Global Stem Cells Group Announces New President of the International Society for Stem Cell Application (ISSCA)
Miami, Florida – Global Stem Cells Group, a leading organization in the field of regenerative medicine, is pleased to announce the appointment of Dr. Salih Yildirim as the new President of the International Society for Stem Cell Application (ISSCA), a division of Global Stem Cells Group. Dr. Yildirim previously held the position of Director of Overseas Operations at ISSCA.
Dr. Salih Yildirim brings a wealth of experience and expertise to his new role as President of ISSCA. Having previously served as the Director of Overseas Operations, Dr. Yildirim has demonstrated his exceptional leadership skills and strategic vision throughout his career. He has played a vital role in expanding ISSCA’s international presence and fostering collaborations with stakeholders worldwide.
In a statement regarding the appointment, Dr. Daeyong Kim, the former President of ISSCA, expressed his confidence in Dr. Yildirim’s ability to lead the organization. Dr. Kim stated, “I think you are the best leader and the most prepared to be the President of ISSCA. Therefore, I think you should be the head of ISSCA.” Dr. Kim will now transition to other support functions within ISSCA.
Benito Novas, head of Public Relations for ISSCA, expressed his admiration for Dr. Salih Yildirim’s professional qualities. Novas said, “I have known and worked with Dr. Salih for more than 10 years, and I am honored to have him as President within the company. During these years, he has proven to be an exceptional leader with a great vision for ISSCA.”
ISSCA’s objectives under the leadership of Dr. Salih Yildirim will include advancing the understanding and application of stem cell therapies, promoting scientific research and collaboration among professionals in the field, and advocating for the ethical and responsible use of regenerative medicine worldwide. Dr. Yildirim’s extensive experience in business development, international relations, and regenerative medicine will undoubtedly contribute to the achievement of these objectives.
About Dr. Salih Yildirim:
Dr. Salih Yildirim holds a bachelor’s and master’s degrees in business administration from Cleveland State University (United States) and a Ph.D. in health management from the University of Health Sciences. He has held various key positions throughout his career, including Director of Overseas Operations at ISSCA. Dr. Yildirim is currently the General Manager of BioTrend Medical International, a biotechnology firm specializing in genetics, molecular biology, and stem cell technologies. He also serves as the chief executive officer of ReGen.IC Clinic, specializing in regenerative medicine and stem cell applications. Additionally, he is a member of the International Society for Stem Cell Application’s Board of Directors and the Global Stem Cell Group’s Board of Directors and International Operations Manager. Dr. Yildirim is also a member of Istanbul Atlas University’s Board of Directors. He is married and a proud father.
About ISSCA
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians who aspire to treat diseases and lessen human suffering through advances in science, technology, and regenerative medicine.
ISSCA updates its members on advances in stem cell research, MSC, exosomes, and regenerative medicine
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
The international community provides a platform for practitioners to interact with scientists and build medical networks necessary for marketing.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities worldwide. The goal is to encourage more physicians to practice regenerative medicine and make it available to patients nationally and internationally. Incorporated under the Republic of Korea as a non-profit company, the ISSCA is focused on fostering excellence and standards in regenerative medicine.
About Global Stem Cells Group
Global Stem Cells Group is the parent company of six companies that are dedicated to stem cell research, solutions, and technology training. The group was founded in 2012 and combines dedicated researchers, patient educators, and physician trainers with the shared goal of meeting the need for high-end stem cell solutions and treatments.
Given that the group has a singular focus in this field, it is uniquely positioned to become the global leader in cellular medicine. In addition, by bringing together leading professionals in cellular medicine, it can discover issues that the industry faces and focus its research and development in this area. This specialization has, undoubtedly, enabled it to come up with solutions that address some of the significant issues that most stakeholders are facing in the industry.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
Global Stem Cells Group (GSCG) and International Society for Stem Cell Application (ISSCA) to Participate in Dubai Stem Cell Congress 2023
MIAMI, Florida, February 15, 2023 – Global Stem Cells Group (GSCG) and its educational division, the International Society for Stem Cell Application (ISSCA), are proud to announce their participation as sponsors and co-organizers of the Dubai Stem Cell Congress 2023. The congress, endorsed by the Hortman Stem Cell Laboratory, will take place on the 27th and 28th of February 2023 at the prestigious Waldorf Astoria on The Palm in Dubai.
The congress will bring together international experts in regenerative medicine and stem cell research from around the world to share their knowledge, experiences, and insights with the academic community and the general public. The event will also provide a platform for patients to share their success stories of stem cell therapy and how it has helped them overcome various life-threatening diseases.
“We are honored to welcome you to the first edition of the Dubai Stem Cell Congress 2023, which will host international experts in the field of Regenerative Medicine & Stem Cell Research from around the world,” said Dr. Fatma Alhashimi, Chairman of Dubai Stem Cell Congress. “We will be showcasing cutting-edge data, stem cell technologies, and advanced applications.”
GSCG will showcase its range of cellular products and equipment, including exosomes for topical and intravenous use, Mesenchymal Stem Cells (MSC), Bone Marrow Kit, PRP Kit, Fat Kit for obtaining Stromal Vascular Fraction (SVF), workstation, and laboratory equipment. ISSCA, on the other hand, will be represented with its training, congresses, conferences, and the fellowship in Cellular Therapies.
“Our mission is to make cellular therapies a reality for both doctors and patients, and we are happy to continue that mission by sharing our knowledge and showcasing our Cellgenic branded Cellular products and equipment at the congress,” said Benito Novas, Managing Director of Global Stem Cells Group and Head of Public Relations for ISSCA.
With over 25 world-class international speakers, including ISSCA-certified speakers, the congress will feature a variety of panel discussions, lectures, and presentations on the latest innovative technologies in stem cell and regenerative medicine.
ISSCA-certified speakers and experts who will be presenting lectures at the congress include the following:
- Erdinç Civelek, a brain surgeon at Gaziosmanpaşa Taksim Hospital for Research and Education in Turkey.
- Dr. Serdar Kabataş, a brain surgeon at Gaziosmanpaşa Taksim Research and Education Hospital in Turkey.
- Abdulmajeed Hamadi, a consultant hematologist and pioneer in transplantation and stem cell therapy with an FRCP designation in Iraq.
- Salih Yildirim, the Director of Overseas Operations of ISSCA and a member of the Board of Directors of the Global Stem Cell Group and Director of International Operations.
- Benito Novas, the Managing Director of Global Stem Cells Group and Head of Public Relations for the International Society for the Application of Stem Cells in the USA.
Among the esteemed speakers at the event are:
Prof. Anil Dhawan, Dr. Frances Verter, Dr. Masayo Takahashi, Dr. Essam Abdelalim, Prof. Shuibing Chen, Prof. John E. Wagner, Mrs. Kim Petrella, Dr. Edward Guindi, Dr. Mazaiah Yaacob, Dr. Mohammed Moulay, Mr. Oliver Papavlassopoulos, Dr. Sean Ng, Dr. Chiara Cugno, Dr. Siti Aminah, Mr. Thomas Moss, Prof. Dr. Tim Schulz, Dr. Luis Saraiva, Mrs. Renata Mihályová, Dr. Srinivasan Periathiruvadi and Dr. Ahmed Foul.
For the first time in the UAE, patients will have the opportunity to share their success stories of stem cell therapy, including the story of Mahra, a UAE national and survivor of Thalassemia, and the world’s first cord blood patient, Matthew Farrow.
Stem cell therapy is the present and future of medicine and holds the “Hope That Brings Life” to many patients in need. The Dubai Stem Cell Congress 2023 is a landmark event that will bring together leading experts, patients, and members of the academic community to share their experiences and knowledge about stem cell therapy and its advancements.
To learn more about the Dubai Stem Cell Congress 2023 and to make a reservation, visit the https://dubaistemcellcongress.com/ website, email info@stemcellsgroup.com, or call +1 305 560 5337.
About Hortman Stem Cell Laboratory
Mr. Darius Curta Managing Director of Hortman Healthcare Investment – Hortman Stem Cell Laboratory is the first state-of-the-art laboratory within the field of Stem Cells and Regenerative Medicine, focused on providing top-notch cord blood banking solutions. Additionally, our institution will be facilitating the first ever clinical trials in stem cell research in UAE. Hortman Stem Cell Laboratory, located at Golden Mile, Palm Jumeirah, consists of GMP Laboratories & ISO Clean Rooms for stem cell isolation, culture & expansion. Such a facility enables us to provide a solid foundation for successfully establishing an awareness and understanding of stem cells within the Middle Eastern Region. Hortman is leading that charge at the forefront of the research community.
About ISSCA:
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians who aspire to treat diseases and lessen human suffering through advances in science, technology, and the practice of regenerative medicine. ISSCA serves its members through advancements made in the specialty of regenerative medicine.
The mission of the International Stem Cell Certification Agency (ISSCA) is to establish itself as a global leader in regenerative medicine certification, education, research, and training.
ISSCA provides certification training in cities worldwide because it recognizes the importance of standards and certifications in regenerative medicine as a medical specialty. To help more people, both locally and globally, as the demand for more doctors interested in and comfortable with regenerative medicine surges. ISSCA’s mission is to advance quality and uniformity in regenerative medicine worldwide.
About Global Stem Cells Group:
The Global Stem Cell Group is a family of several companies focused on stem cell medicine and research. The company uses its network to bring leadership in regenerative medicine training, research, and patient applications.
GSCG’s mission is to allow physicians to present the benefits of stem cell medicine to patients worldwide. The company also partners with policymakers, educators, and regulators to promote regenerative medicine.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website www.stemcellsgroup.com or call +1 305 560 5331
Safe Harbor Statement:
Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
ISSCA to Conduct Regenerative Medicine Conference in Cancún, México
Miami, FL, December 19, 2022 – The International Association for Stem Cell Application (ISSCA) has announced plans to host Applications of Stem Cell Therapies in Medicine and Aesthetic Surgery, a regenerative medicine conference, in Cancun, Mexico, April 22, 23, and 24, 2023.
Growing knowledge can turn the tide in the clinical and translational spectrum of stem-cell-based research and therapy, and several obstacles must be overcome. ISSCA’s International Conference, champions new ideas to propel the shift from traditional healthcare to regenerative medicine and therapies. The Conference will also emphasize the role of cutting technology and developments in all areas of stem cell research.
April 22nd & 23rd
The Cancun event will feature a panel of internationally renowned experts on stem cells and regenerative medicine, who will offer a two-day rigorous scientific discourse on critical topics in stem cell research and clinical applications. Topics of focus at the conference include: Tissue engineering and regenerative medicine; updates in Stem Cell research Clinical applications of regenerative medicine and cellular therapies; the latest methods of harvest and isolation, and Non-invasive surgical protocols; strategies for successful delivery of cell products; Ethical and regulatory issues in stem cell technology; and taking advantage of the latest technologies to improve marketing and networking.
April 24th
The third day of the conference (April 24th) will involve intensive hands-on training sessions with ISSCA-certified instructors on stem cell therapy’s aesthetic and clinical applications.
Participants will gain experience and access to different technologies, brands, and products for stem cell therapy.
The leading international conference offers an excellent opportunity for medical practitioners and stakeholders to network and learn from the best in the industry.
All practitioners and stakeholders in the medical sector are invited, including:
- Doctors Researchers & innovators,
- Decision-makers and funding agencies,
- Experts seeking collaborative work on stem cell technology,
- Professional bodies, and
- Marketing professionals.
To learn more about the ISSCA Cancun, Mexico conference and to make a reservation, visit the https://www.issca.us/issca-world-conferences/ website, email info@stemcellsgroup.com, or call +1305 560 5337.
About ISSCA
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians who aspire to treat diseases and lessen human suffering through advances in science, technology, and regenerative medicine.
ISSCA updates its members on advances in stem cell research, MSC, exosomes, and regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
The international community provides a platform for practitioners to interact with scientists and build medical networks necessary for marketing.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities worldwide. The goal is to encourage more physicians to practice regenerative medicine and make it available to patients nationally and internationally. Incorporated under the Republic of Korea as a non-profit company, the ISSCA is focused on fostering excellence and standards in regenerative medicine.
About Global Stem Cells Group
Global Stem Cells Group is the parent company of six companies that are dedicated to stem cell research, solutions, and technology training. The group was founded in 2012 and combines dedicated researchers, patient educators, and physician trainers with the shared goal of meeting the need for high-end stem cell solutions and treatments.
Given that the group has a singular focus in this field, it is uniquely positioned to become the global leader in cellular medicine. In addition, by bringing together leading professionals in cellular medicine, it can discover issues that the industry faces and focus its research and development in this area. This specialization has, undoubtedly, enabled it to come up with solutions that address some of the significant issues that most stakeholders are facing in the industry.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
- Published in Press Releases
Announcement of Training in Fort Lauderdale, Florida by The Global Stem Cells Group
Recently, the Global Stem Cells Group announced plans to teach physicians the value and process behind incorporating regenerative medicine into their own clinical practices. MIAMI, 4–5 November 2022—Global Stem Cells Group, a multidisciplinary community of scientists and physicians that are working together to cure diseases and relieve human pain through the advancement of the field of regenerative medicine, announced today a plan of training in Fort Lauderdale on November 4th and 5th. This training aims to equip physicians with the value and knowledge behind incorporating regenerative medicine into their own clinical practice. Benito Novas, CEO of GSCG, is confident that the event will bring together a group of:
- Doctors are seeking training, products, and equipment in regenerative medicine.
- Doctors who care for patients with conditions that can be treated with cell therapies
- Aesthetic doctors.
- Doctors who want to be up to date with the latest technologies and protocols
- Doctors with an interest in new research on stem cells, MSC, Exosomes, and medical networks want to attract more clients.
The training course is intended to cover:
- Hands-On portion: Doctors, in a controlled environment and guided by a team of medical professionals, will have the opportunity to see procedures being performed a few feet away and then get the opportunity to try them for themselves.
- Review of stem cell biology
- Characterization of cells, cell products, cytokines, and growth factors, as well as their capacity for regeneration.
- Laboratory Processes
- Clinical applications
- Product validation
- Practice Management
- Patient acquisition
The International Society for Stem Cell Application (ISSCA) has done intensive research on this topic and the team will take a lead in educating, training, and certification during the event. As a medical speciality, regenerative standards and certification are very important, which is why ISSCA will provide certificates after the training. By the end of the training course, you will understand everything you need to know to add adult stem cellbased procedures to your existing practice. As before, the united efforts of the seven major medical corporations will provide practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards. To find more about the courses and to make reservations for this particular event, head on to their website www.issca.us/autologous-miami-november/ .us email info@stemcellsgroup.com or dial +1305 560 5337.
About ISSCA
ISSCA is a multidisciplinary community that brings together scientists and physicians, all of whom aspire to treat diseases and relieve human pain through advances in science, technology, and the practice of regenerative medicine. ISSCA serves its members through innovations made to the speciality of regenerative medicine. The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, training, research, education, and certification.
As a medical speciality, regenerative medicine standards and certifications are very important, which is why ISSCA provides certification training globally. This is because they want to encourage more physicians to practice regenerative medicine and make it accessible to benefit patients both nationally and worldwide. Incorporated under the Republic of Korea as a nonprofit entity, the ISSCA’s main goal is to promote excellence and standards in the field of regenerative medicine. As a physician, missing this chance to book a personalized hands-on training session must cost you dearly. Contact + 1 305 560 5337 to book as early as possible.
About Global Stem Cells Group
Global Stem Cells Group is the parent company of six companies that are dedicated to stem cell research, solutions, and technology training. The group was founded in 2012 and combines dedicated researchers, patient educators, and physician trainers with the shared goal of meeting the need for high-end stem cell solutions and treatments.
Given that the group has a singular focus in this field, it is uniquely positioned to become the global leader in cellular medicine. In addition, by bringing together leading professionals in cellular medicine, it can discover issues that the industry faces and focus its research and development in this area. This specialization has, undoubtedly, enabled it to come up with solutions that address some of the significant issues that most stakeholders are facing in the industry.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
- Published in Press Releases
Global Stem Cells Group Announces the Launch of a New Bone Marrow Harvesting Kit
The Global Stem Cells Group has announced the launch of a new bone marrow harvesting kit in response to a request for effective and accessible solutions to regenerative medicine procedures
Bone marrow is a spongy tissue that is located inside most large bones – it is essential for generating red blood cells (erythrocytes), white blood cells (leukocytes) and platelets (thrombocytes), using a bone marrow aspirate needle to collect the aspirate can help physicians perform different regenerative treatments especially in osteomioarticular conditions.
Increased Stem and Progenitor Cell Concentrations in Marrow Aspiration
According to the company, the new kit for processing and extraction of pure bone marrow has demonstrated its ability to increase stem and progenitor cell concentrations in marrow aspiration compared to traditional needles. It further adds that the device overcomes the limitations of traditional needles that restrict the amount of peripheral blood that would otherwise have been aspirated by forcing aspiration flow through side ports rather than from an open cannula. This setup has been a major issue with traditional needles and often led to the extraction of excessive amounts of bone marrow as well as impure marrow.
“Cellgenic plans with this new device to position itself as a leader in the development and manufacture of single-use medical devices for a number of medical specialties, we have developed a new bone marrow aspiration and biopsy needle that is manual, sterile and disposable, allowing precise procedures to be performed” said Benito Novas C.E.O of Global Stem Cells group
The new system offers the facility for doctors to reposition the needle more precisely at different access points. Precision is vital when trying to access bone marrow from specific sections of the bone.
According to Benito Novas, CEO of Global Stem Cells Group, the product will be launched at a press conference this September in Istanbul, Turkey within the framework of the World Congress of Regenerative Medicine. Additionally, it will be available at retail for doctors and distributors worldwide from October 2022
Buyers will undergo an orientation to use the new kit. It is easy to work with and many are expected to start using it as soon as they purchase it. The stem cells group will offer all the necessary training and support for the doctors during training and thereafter to ensure that the medical practitioners enjoy the full benefits of the device.
Global Stem Cells Group has been on the frontline in offering quality devices for stem cell procedures. It aims to make procedures as simple, risk-free, and efficient as possible for the maximum benefit of patients.
About Global Stem Cells Group
Global Stem Cells Group is the parent company of six companies that are dedicated to stem cell research, solutions, and technology training. The group was founded in 2012 and combines dedicated researchers, patient educators, and physician trainers with the shared goal of meeting the need for high-end stem cell solutions and treatments.
Given that the group has a singular focus in this field, it is uniquely positioned to become the global leader in cellular medicine. In addition, by bringing together leading professionals in cellular medicine, it can discover issues that the industry faces and focus its research and development in this area. This specialization has, undoubtedly, enabled it to come up with solutions that address some of the significant issues that most stakeholders are facing in the industry.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
- Published in Press Releases
Global Stem Cells Group to Represent CELLINK 3D Bioprinting Technology in Latin America

Global Stem Cells Group announces that it will represent CELLINK Bioprinting Technology in Latin America. CELLINK is the world’s first company to market bioinks for 3D bioprinting of human organs and tissue.
MIAMI, Nov. 9, 2017—Global Stem Cells Group (GSCG), a world leader in stem cell and regenerative medicine, announces it will represent CELLLINK 3D Bioprinting Technology in Latin America. CELLINK is the world’s first company biotech company to market bioinks for 3D bioprinting of human organs and tissue.
GSCG will also market CELLINK’S newest product, BIO X printer for researchers, life science companies, and innovators who work with bioprinting on its subsidiary Adimarket.net website.
 3D bioprinting of human tissues and organs is a revolutionary technology in the field of tissue engineering. One of the major challenges in stem cell research and tissue engineering is mimicking the micro and macro environment of human tissues. A favorable functional outcome is extremely dependent on the level to which tissue scientist and engineers are able to control the inner micro- and macro-scale features of engineered-tissue. In response to this challenge, advances in additive manufacturing inspired scientists to develop and adapt 3D bioprinting technology for human tissues and organs.
3D bioprinting of human tissues and organs is a revolutionary technology in the field of tissue engineering. One of the major challenges in stem cell research and tissue engineering is mimicking the micro and macro environment of human tissues. A favorable functional outcome is extremely dependent on the level to which tissue scientist and engineers are able to control the inner micro- and macro-scale features of engineered-tissue. In response to this challenge, advances in additive manufacturing inspired scientists to develop and adapt 3D bioprinting technology for human tissues and organs.
“Our objective is to bring this cutting-edge 3D bioprinting technology to scientists and regenerative medicine researcher throughout Lain America,” says Global Stem Cells Group founder and CEO Benito Novas. “CELLINK has revolutionized tissue engineering with its range of bioprinters, and we’re excited to make this process available to scientists and regenerative medicine researchers in Mexico, Central America, and South America.
“This opportunity is ideally suited to Global Stem Cells’ commitment to advancing the benefits of stem cell medicine in Latin America and worldwide, Novas says.”
To learn more about Global Stem Cells Group, visit the GSCG website, email info@stemcellsgroup.com, or call +1305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
About AdiMarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a one-stop, cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide practitioners the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting-edge treatments.
###
CELLLINK 3D bioprinting technology
- Published in Press Releases
Texas Man Becomes First Adult in the U.S. to Receive Updated Stem Cell Transplant to Treat Leukemia
Introduction to the Groundbreaking Stem Cell Transplant
Chuck Dandridge, a Mansfield, Texas resident, became the first adult in the U.S. to receive a newly modified stem cell transplant that uses genetically engineered blood cells from a family member. The milestone was announced by researchers at UT Southwestern Medical Center’s Harold C. Simmons Comprehensive Cancer Center in Dallas, where the procedure was performed.
Dandridge’s Medical Journey and Diagnosis
Dandridge’s medical journey began in 2013, with a routine doctor’s visit to check his cholesterol levels; lab tests revealed low blood counts and further testing confirmed Dandridge’s diagnosis of myelodysplastic syndrome, also called pre-leukemia or MDS. By 2014, the leukemia had progressed to acute myeloid leukemia (AML), which, according to the National Cancer Institute, affects more than 20,000 Americans annually.
Genetic Testing and Clinical Trials

Dandridge was referred to UT Southwestern’s Simmons Cancer Center, where his leukemia was tested for genetic mutations.
“We wanted to know whether he had specific mutations in his cancer cells,” says Madhuri Vusirikala, M.D., Professor of Internal Medicine and the primary investigator of many UT Southwestern clinical trials related to bone marrow transplantation. “We found a mutation called IDH 2, which causes the body to produce an abnormal protein that promotes excessive cell growth. If you can target that mutation and stop the abnormal protein from being produced, then cells start behaving normally.”
Participation in the AG-221 Clinical Trial
Dandridge enrolled in a UT Southwestern clinical trial for a therapy called AG-221. He took four pills each morning for the next eight months. During that time, Dandridge saw marked improvement although he did not go into complete remission, according to Vusirikala. That success made him eligible for a potentially curative stem cell transplant. But finding a donor proved challenging.
Challenges in Finding a Suitable Donor
“The best chance of finding a full match is usually a full sibling; however, Chuck has no full siblings,” Vusirikala says. Additionally, Dandridge is African American, and minorities are under-represented in the National Marrow Donor Registry—about 70 percent of registry donors are Caucasian. The search for an unrelated donor was unsuccessful. Vusirikala says that he knew Dandridge’s daughter and his son would be at least a half match. Since using a same-sex donor is preferred, as it reduces the risk of complications, his son Jon, 31, emerged as the best choice. But the risk of graft-versus-host-disease (GvHD) following a transplant using a half-match is very high, so they needed a better way to deal with the GvHD risk.
Innovative BP-001 Clinical Trial

Once again, Mr. Dandridge volunteered for a cutting-edge clinical trial, known as BP-001, which processed the stem cells used in the transplant to reduce the risk of rejection and engineered blood cells that can be targeted if GvHD develops after the transplant. The processes being tested in BP-001 are in clinical development by Houston-based Bellicum Pharmaceuticals. The study is evaluating patients with blood cell cancers who have a peripheral blood stem cell transplant from a partially matched relative. Immune cells (T cells) from the related donor are separated from the rest of the stem cells and genetically engineered in the Bellicum laboratory, and then given to the patient along with the stem cell transplant.
Procedure and Outcome of the Transplant
These engineered T cells are modified to include a suicide gene with the help of a retrovirus. If the patient develops GvHD after transplant, the side-effect can be treated by giving a drug called rimiducid to activate the suicide gene and cause the activated GvHD-causing cells to be eliminated. The stem cells given for the transplant were also processed prior to giving them back to Dandridge to reduce the risk of graft rejection as well as GvHD. The genetically engineered blood cells were transplanted from Dandrige’s son, Jon, 31, to the father in three, two-hour infusions at William P. Clements Jr. University Hospital in July 2015, and today the elder Mr. Dandridge’s leukemia is in remission. His immune system is recovering, and the former Norman, Oklahoma YMCA CEO is now mentoring first-time CEOs for the YMCA.
- Published in Corporate News / Blog
Amazing Stem Cell Research Breakthroughs You Never Heard of
Introduction to Skin Stem Cell Research
Stem cell research has uncovered numerous groundbreaking discoveries over the years, many of which remain relatively unknown. This article highlights one such discovery related to skin stem cells and their critical role in regeneration and maintenance.
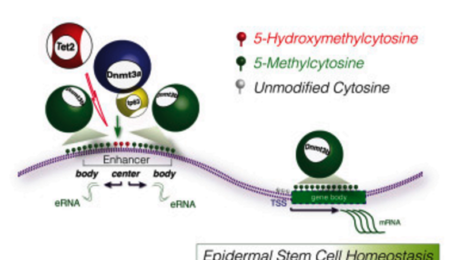
Discovery of Dnmt3a and Dnmt3b Proteins
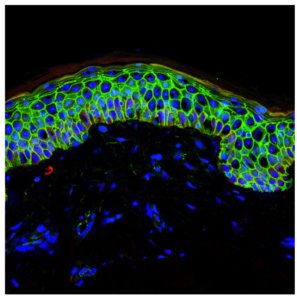
In a significant 2008 study published in “Cell Stem Cell,” researchers from the Catalan Institution for Research and Advanced Studies (CREA) identified two proteins—Dnmt3a and Dnmt3b—that play a crucial role in maintaining skin stem cells. Led by Salvador Aznar Benitah at the Institute for Research in Biomedicine (IRB Barcelona), the study revealed that these proteins are essential for the activation and preservation of skin stem cells.
Function of Dnmt3a and Dnmt3b in Skin Stem Cells

According to Benitah, head of the Stem Cells and Cancer lab at IRB Barcelona, without Dnmt3a and Dnmt3b, skin stem cells fail to activate and eventually diminish within the tissue. The proteins operate primarily on gene enhancers and super-enhancers, facilitating the expression of approximately 1,000 genes critical for the self-renewal of skin stem cells.
Genomic Insights and Mechanisms
Lorenzo Rinaldi, a researcher involved in the study, utilized advanced sequencing techniques to map the genomic distribution of Dnmt3a and Dnmt3b. This revealed their unexpected role in enhancing gene expression through DNA methylation, contrary to their previously known function in gene repression.
Link to Cancer Research
The study also highlighted implications for cancer research, noting that Dnmt3a and Dnmt3b are altered in various types of tumors, including leukemia, lung cancer, and colon cancer. The proteins’ role in DNA methylation and gene regulation suggests potential contributions to tumor development, warranting further investigation in cancer cell models.
Funding and Support
Funded by the Spanish Ministry of Economy and Competitiveness and supported by several foundations and councils, including The European Council for Research (ERC) and the Fundació Marató de TV3, Benitah’s research underscores the importance of public and private partnerships in advancing stem cell and cancer research.
Conclusion
This study represents a significant advancement in understanding the molecular mechanisms governing skin stem cell maintenance and its implications for both regenerative medicine and cancer biology. Continued research into Dnmt3a and Dnmt3b promises to unveil new therapeutic strategies and insights into cellular regeneration and disease progression.
- Published in Corporate News / Blog
New Guidelines for Stem Cell Research and Therapies Aim to Protect Patients from Charlatan Quackery
Introduction
Stem cell research has advanced significantly, leading to a myriad of treatment options. However, the field faces challenges from unscrupulous providers and premature publicity.
Professional Guidelines for Responsible Stem Cell Research
International Society for Stem Cell Research (ISSCR)

The ISSCR leads in setting high standards for translational stem cell research:
- Emphasizes rigorous preclinical evidence and peer review.
- Highlights the importance of IRB review and comprehensive informed consent.
International Society for Cellular Therapy (ISCT)
The ISCT expands its scope beyond stem cells, advocating for broader cell-based interventions:
- Focuses on defining scientific evidence and regulatory practices.
- Addresses implications across clinical practice and commercialization.
Development of New Guidelines
Terminology and Scientific Evidence
Efforts are underway to standardize terminology and define scientific evidence levels critical for ethical and effective stem cell therapy.
ISSCR’s 2016 Guidelines Update
In 2016, ISSCR updated guidelines cover:
- Emerging technologies like gene editing and induced pluripotent stem cells (iPSCs).
- Upholding ethical standards such as the “14-day rule” for embryo experimentation.
Key Topics Addressed in the Revised Guidelines
The updated guidelines include:
- Oversight processes for embryo research and mitochondrial replacement therapy.
- Standards for preclinical and clinical research, emphasizing safety and efficacy.

Advancements in Stem Cell Research
Stem cell therapies show promise in treating a range of conditions, leveraging pluripotent stem cells for tissue repair and genetic disease treatments.
Conclusion
Stem cell research continues to evolve responsibly, offering hope for future medical advancements while safeguarding patient interests against fraudulent practices.
- Published in Corporate News / Blog
Researchers Move Closer to Lung Stem Cell Therapies to Treat Chronic Lung Diseases
Introduction
Chronic lung diseases, including COPD, bronchitis, emphysema, and asthma, are significant causes of mortality in the U.S., highlighting the urgent need for advanced treatments.
Stem Cells in the Lung
Types and Functions of Lung Stem Cells
Human lungs are complex organs comprising:
- Conducting Airway Tubes: Includes trachea, bronchi, and bronchioles.
- Gas Exchange Regions: Alveolar spaces crucial for oxygen exchange.
Role of Progenitor Cells
Progenitor cells like tracheal basal cells and alveolar type 2 cells play a vital role in maintaining lung health by replacing old or damaged cells.
Diversity of Lung Stem Cells
Embryonic and adult lung stem cells contribute differently:
- Research indicates their role in lung development and regeneration, with potential implications for disease treatment.
Current Research on Lung Stem Cell Therapies
Adult Mesenchymal Stem Cells (hMSCs)
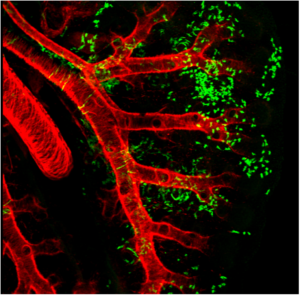
hMSCs are pivotal in:
- Immunomodulation: Regulating immune functions and secreting bioactive molecules for anti-inflammatory and regenerative effects.
- Versatility: Capable of generating various cell types, potentially aiding in lung tissue repair.
Insights into Lung Disease Causes
Understanding lung stem cell biology enhances knowledge of disease mechanisms like COPD, paving the way for innovative treatments.

Future Prospects of Lung Stem Cell Therapies
Translational Research and Clinical Applications
Ongoing studies aim to:
- Identify and characterize lung stem cells in humans, advancing potential clinical applications.
- Explore personalized medicine approaches using lung stem cells for targeted therapies.
Conclusion
Progress in lung stem cell research holds promise for developing effective therapies to combat chronic lung diseases, offering hope for improved patient outcomes and quality of life.
- Published in Corporate News / Blog
- 1
- 2