New advances in osteoarthritis therapy with stem cells
Osteoarthritis is a rheumatic pathology that damages the articular cartilage. By joining two bones through the joint capsule, the joints are able to move, providing us with functional autonomy. An inner fluid called synovial fluid is usually found within joints, which is produced by the synovial membrane. Articular cartilage covers the ends of the bones that form the joint. As a result of damage to this articular cartilage, pain, stiffness, and functional impairment occur. Osteoarthritis is the most common joint disorder, usually beginning between the ages of 40 and 50, affecting to some degree almost everyone over the age of 80. Typically, osteoarthritis affects the spine, shoulders, fingers, hips, knees, and toe joints.
Stem cell therapies have the potential to treat a broad spectrum of diseases. Whether it’s rhizarthrosis, diabetes, neurodegenerative diseases, spinal cord injuries, or heart disease. By utilizing stem cells, regenerative medicine is capable of repairing tissues in affected areas. The main difference between lipo gem therapy and other treatments for osteoarthritis is that lipogem therapy regenerates cartilage, avoids surgery and its sequelae, and improves the quality of life for patients.
The potential for medical treatments with stem cells and their by-products is currently very high. In the field of sports medicine and traumatology, one of the most outstanding advances has been made for the first time in decades recently: Spanish scientists have achieved a degree of tendon regeneration in 100% of injured patients, resulting in a decrease in pain and a return to sport within two months, and just six months after the trial was completed.
A research performed by the Institute of Regenerative Tissue Therapy (ITRT), published by the prestigious American Journal of Sports Medicine. Demonstrates how this therapy regenerates chronic lesions in the patellar tendon and opens up a new therapeutic option for this tissue, which was considered impossible to regenerate.
In most patients, fat tissue can be harvested minimally invasively (under local or general anesthesia), providing a highly viable MSC population regardless of donor age. Similar to MSCs derived from other tissues, adipose tissue-derived MSCs have regenerative potential. As osteoarthritis is a very common joint disease, and knee osteoarthritis is the most common form, it is necessary to review scientific literature on osteoarthritis treatments with stem cells, like lipogems.
Lipogems Therapy
Lipogems therapy is a novel procedure that enhances the body’s natural ability to heal itself through the innovative power of science and biotechnology. The Lipogems method involves injecting mesenchymal stem cells into the joints. Adipose-derived mesenchymal stem cells have enormous regenerative potential. They also have a regenerative capacity independent of their age. Even older individuals can benefit from this procedure.
Injection of mesenchymal stem cells into the knee, particularly in the early stages of osteoarthritis, can stop the process of inflammation and degeneration, especially in the less advanced stages of the disease. In addition to preventing progressive physical deterioration of the articular cartilage, this treatment contributes significantly to a patient’s well-being and prevents the installation of knee prostheses.
Patellar tendinopathy, physiotherapeutic treatment and stem cell therapy
Injuries to the patellar tendon that connects the kneecap to the tibia are known as patellar tendinopathy or patellar tendinitis. The patellar tendon works with the muscles in the front of the thigh to extend the knee so you can kick, run and jump. Athletes who perform frequent jumping in their sports, such as basketball and volleyball, are most likely to suffer from patellar tendonitis. However, people who don’t engage in jumping sports may develop patellar tendonitis. Patients with patellar tendinitis usually begin treatment with physical therapy to stretch and strengthen their knee muscles.
Strength training with eccentric resistance is one of the most common treatments for tendinopathies. Alternatively, it has been demonstrated that bone marrow-derived mesenchymal stem cells (MSCs) can regenerate injured patellar tendons. Within six months of treatment, it has been observed that the structure of this tissue – which is always difficult to treat – is restored, reaching a regeneration of 40% in all injured persons, with a gradual improvement that eventually becomes complete.
It has been found that traditional management methods, including isometric or eccentric exercises, shock wave therapy, and even surgery, are not effective. As part of a rehabilitation program in chronic patellar tendinopathy, autologous expanded bone marrow mesenchymal stem cells (BM-MSC) or leukocyte-poor platelet-rich plasma (Lp-PRP) may be effective in reducing pain and improving activity levels. Traditional management, which includes isometric or eccentric exercises, shock wave therapy, and even surgery, has limited success. A combination of autologous expanded bone marrow mesenchymal stem cells (BM-MSCs) and leukocyte-poor platelet-rich plasma (Lp-PRP) and rehabilitation may reduce pain and improve activity levels in active participants with chronic patellar tendinopathy.
To learn more about stem cells, cellular therapies and keep up to date with all the information about regenerative medicine and its advances, sign up for our international certification in regenerative medicine at www.issca.us
- Published in Corporate News / Blog
Global Stem Cells Group (GSCG) and Dr. Yanti Aesthetic Clinic Elevate Regenerative Medicine Landscape with New Stem Cell Center in Jakarta
Miami, Florida – Global Stem Cells Group (GSCG) is thrilled to announce the grand opening of its latest state-of-the-art Stem Cell Center, located in Jakarta, Indonesia. This momentous occasion marks a significant stride forward in the field of regenerative medicine and showcases GSCG’s commitment to providing innovative healthcare solutions worldwide.
In a groundbreaking partnership with the esteemed Dr. Yanti Aesthetic Clinic, GSCG introduces its third Stem Cell Center in Indonesia, complementing existing facilities in Surabaya and Bandung. Further underscoring their dedication to advancing medical research, GSCG also anticipates the unveiling of a fourth location in Bali in the near future.
The launch of the Stem Cell Center in Jakarta serves as a beacon of innovation, the International Society for Stem Cell Application (ISSCA), GSCG’s educational division, conducted a groundbreaking training session alongside Dr. Alan Gaveck. This session spotlighted the transformational potential of NK cell therapies in treating Cancer and Anti-aging concerns, further cementing GSCG’s pioneering influence.
A distinctive feature of this collaboration is the authorization granted to Dr. Yanti Aesthetic Clinic to leverage GSCG’s brand, technologies, products, and treatment protocols. Operating as a franchise, the center proudly carries the brand, adhering to meticulous organizational guidelines, while being owned and operated by Dr. Yanti Aesthetic Clinic. This symbiotic arrangement seamlessly merges shared expertise with individual ownership, fostering a harmonious blend of vision and innovation.
Beyond its role as a clinical hub, the newly inaugurated Stem Cell Center will also serve as a focal point for ISSCA’s training initiatives in Asia. In addition to the pioneering NK cell therapy training conducted at the clinic, the center will facilitate educational programs to empower medical professionals across the continent with cutting-edge insights and advancements in regenerative medicine.
Benito Novas, an esteemed figure in the realm of regenerative medicine and the head of Public Relations for GSCG and ISSCA, shared his insights during the inaugural event, stating, “Global Stem Cells Group is dedicated to democratizing regenerative medicine access on a global scale. We encourage physicians to embrace the clinical and aesthetic applications of stem cell therapy, enabling patients around the world to experience the transformative benefits of regenerative treatments.”
Reflecting on the journey that led to this momentous occasion, Novas continued, “Dr. Yanti and I met in Brussels back in 2018, when this ambitious endeavor was but a vision. Today, as we witness the fruition of that vision, I express my profound gratitude for the privilege of collaborating with Dr. Yanti Aesthetic Clinic in ushering in the future of regenerative medicine.”
Dr. Yanti Kushmiran, Director of the DR. Yanti Aesthetic Clinic, echoed these sentiments, stating, “Our partnership with GSCG, a trailblazer with over a decade of pioneering work, resonates deeply with our commitment to excellence. The establishment of our third Stem Cell Center in Indonesia under the esteemed Global Stem Cells Group brand strengthens our ability to provide enhanced services to our patients, ushering in a new era of regenerative healthcare.”
With the inauguration of the Stem Cell Center in Jakarta, GSCG and Dr. Yanti Aesthetic Clinic take a significant stride towards advancing regenerative medicine’s frontiers. This landmark collaboration is poised to shape the future of medical care, leveraging cutting-edge technologies and expertise to benefit patients across the Asian continent and beyond.
About Dr. Yanti Aesthetic Clinic:
Dr. Yanti Aesthetic Clinic is a revered healthcare institution renowned for its state-of-the-art aesthetic and regenerative treatments. With an unwavering dedication to excellence and a forward-thinking approach, Dr. Yanti Aesthetic Clinic is poised to shape the future of regenerative medicine in Indonesia and beyond.
About the International Society for Stem Cell Application (ISSCA):
The International Society for Stem Cell Application (ISSCA) serves as the educational arm of Global Stem Cells Group (GSCG), dedicated to promoting knowledge and expertise in stem cell therapy and regenerative medicine. ISSCA empowers medical professionals across the globe with essential insights and advancements in the field.
About Global Stem Cell Group
The Global Stem Cell Group is a family of several companies focused on stem cell medicine and research. The company uses its network to bring leadership in regenerative medicine training, research, and patient applications.
GSCG’s mission is to allow physicians to present the benefits of stem cell medicine to patients worldwide. The company also partners with policymakers, educators, and regulators to promote regenerative medicine.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website www.stemcellsgroup.com or call +1 305 560 5331
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
Global Stem Cells Group Announces New President of the International Society for Stem Cell Application (ISSCA)
Miami, Florida – Global Stem Cells Group, a leading organization in the field of regenerative medicine, is pleased to announce the appointment of Dr. Salih Yildirim as the new President of the International Society for Stem Cell Application (ISSCA), a division of Global Stem Cells Group. Dr. Yildirim previously held the position of Director of Overseas Operations at ISSCA.
Dr. Salih Yildirim brings a wealth of experience and expertise to his new role as President of ISSCA. Having previously served as the Director of Overseas Operations, Dr. Yildirim has demonstrated his exceptional leadership skills and strategic vision throughout his career. He has played a vital role in expanding ISSCA’s international presence and fostering collaborations with stakeholders worldwide.
In a statement regarding the appointment, Dr. Daeyong Kim, the former President of ISSCA, expressed his confidence in Dr. Yildirim’s ability to lead the organization. Dr. Kim stated, “I think you are the best leader and the most prepared to be the President of ISSCA. Therefore, I think you should be the head of ISSCA.” Dr. Kim will now transition to other support functions within ISSCA.
Benito Novas, head of Public Relations for ISSCA, expressed his admiration for Dr. Salih Yildirim’s professional qualities. Novas said, “I have known and worked with Dr. Salih for more than 10 years, and I am honored to have him as President within the company. During these years, he has proven to be an exceptional leader with a great vision for ISSCA.”
ISSCA’s objectives under the leadership of Dr. Salih Yildirim will include advancing the understanding and application of stem cell therapies, promoting scientific research and collaboration among professionals in the field, and advocating for the ethical and responsible use of regenerative medicine worldwide. Dr. Yildirim’s extensive experience in business development, international relations, and regenerative medicine will undoubtedly contribute to the achievement of these objectives.
About Dr. Salih Yildirim:
Dr. Salih Yildirim holds a bachelor’s and master’s degrees in business administration from Cleveland State University (United States) and a Ph.D. in health management from the University of Health Sciences. He has held various key positions throughout his career, including Director of Overseas Operations at ISSCA. Dr. Yildirim is currently the General Manager of BioTrend Medical International, a biotechnology firm specializing in genetics, molecular biology, and stem cell technologies. He also serves as the chief executive officer of ReGen.IC Clinic, specializing in regenerative medicine and stem cell applications. Additionally, he is a member of the International Society for Stem Cell Application’s Board of Directors and the Global Stem Cell Group’s Board of Directors and International Operations Manager. Dr. Yildirim is also a member of Istanbul Atlas University’s Board of Directors. He is married and a proud father.
About ISSCA
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians who aspire to treat diseases and lessen human suffering through advances in science, technology, and regenerative medicine.
ISSCA updates its members on advances in stem cell research, MSC, exosomes, and regenerative medicine
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
The international community provides a platform for practitioners to interact with scientists and build medical networks necessary for marketing.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities worldwide. The goal is to encourage more physicians to practice regenerative medicine and make it available to patients nationally and internationally. Incorporated under the Republic of Korea as a non-profit company, the ISSCA is focused on fostering excellence and standards in regenerative medicine.
About Global Stem Cells Group
Global Stem Cells Group is the parent company of six companies that are dedicated to stem cell research, solutions, and technology training. The group was founded in 2012 and combines dedicated researchers, patient educators, and physician trainers with the shared goal of meeting the need for high-end stem cell solutions and treatments.
Given that the group has a singular focus in this field, it is uniquely positioned to become the global leader in cellular medicine. In addition, by bringing together leading professionals in cellular medicine, it can discover issues that the industry faces and focus its research and development in this area. This specialization has, undoubtedly, enabled it to come up with solutions that address some of the significant issues that most stakeholders are facing in the industry.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
ISSCA launched its first regenerative medicine training in Hormonal Modulation and Gynecology
Miami, Florida – The International Society for Stem Cell Application (ISSCA), a division of Global Stem Cells Group (GSCG), successfully launched its inaugural training course in Hormonal Modulation and Gynecology, marking a significant milestone in the field of regenerative medicine. The course, held from June 10-12, 2023, in Cancun, Mexico, generated immense enthusiasm among participants as the Global Stem Cell Group demonstrated its commitment to disseminating cutting-edge training and information to medical professionals worldwide.
Led by a Well-Respected Doctor
Under the guidance of Dr. Jorge Alberto Elias, a renowned Gynecologist and Urogynecologist with over a decade of experience in Cellular Therapies, participants had the privilege of learning from a highly respected expert in the field. His expertise ensured that attendees received valuable and up-to-date information, enhancing the quality of their educational experience.
The event was divided between two esteemed venues, with the theoretical portion taking place at the Four Points Hotel, and the hands-on training conducted at the prestigious Cellular Hope Institute.
ISSCA commitment to advancing regenerative medicine was further demonstrated by the inclusion of over 30 doctors who joined the event remotely, accessing the conferences and the live proceedings through online channels. This approach enabled medical professionals worldwide to be part of the educational experience, even if they couldn’t attend in person. By embracing technology, the company expanded the reach of knowledge dissemination and fostered a global community of practitioners dedicated to staying at the forefront of regenerative medicine.
The training program focused on equipping doctors and medical professionals with the latest advancements in regenerative medicine, with a specific emphasis on gynecology. Participants delved into the cutting-edge techniques and breakthrough procedures that are shaping the field.
Three Days of Intense Learning
The three-day program was divided into theoretical sessions and hands-on practical training. The theoretical portion took place at the Four Points Hotel, where participants delved into the intricacies of hormonal modulation and its application in gynecology. Dr. Silvina Pastrana, director of ISSCA education in Latam, complemented the program with a session on Exosomes, stem cells, and automatic closed processing techniques using the GCell machine, providing a comprehensive overview of the latest advancements.
During the practical segment, participants convened at the Cellular Hope Institute, where they were immersed in real-life scenarios and witnessed the application of regenerative measures in gynecological cases. Seven diverse cases were presented, including Labiaplasty, Perineum Vaginoplasty, Lichen Sclerosus, Liposuction Mount of Venus, and Lipo fat transfer with open and closed techniques. Through these hands-on demonstrations, attendees gained practical skills and insights into utilizing regenerative medicine to enhance patients’ quality of life.
Reflecting on the success of the event, Benito Novas, Head of Public Relations for the International Society for Stem Cell Application, expressed his enthusiasm, stating, “We are delighted to further expand the ISSCA faculty and specialty areas, opening the door for all physicians to utilize the latest Regenerative Medicine technologies in their area of expertise. Our goal is to provide the best education and training in regenerative medicine and cellular therapies so that professionals can offer the highest quality care to their patients.”
ISSCA celebrates the tremendous success of this inaugural regenerative medicine training course and remains committed to expanding access to the latest knowledge and advancements in the field. By empowering medical professionals globally, ISSCA aims to help patients worldwide benefit from the latest strategies and breakthroughs in regenerative medicine.
About ISSCA
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians who aspire to treat diseases and lessen human suffering through advances in science, technology, and regenerative medicine.
ISSCA updates its members on advances in stem cell research, MSC, exosomes, and regenerative medicine
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
The international community provides a platform for practitioners to interact with scientists and build medical networks necessary for marketing.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities worldwide. The goal is to encourage more physicians to practice regenerative medicine and make it available to patients nationally and internationally. Incorporated under the Republic of Korea as a non-profit company, the ISSCA is focused on fostering excellence and standards in regenerative medicine.
About Global Stem Cells Group
Global Stem Cells Group is the parent company of six companies that are dedicated to stem cell research, solutions, and technology training. The group was founded in 2012 and combines dedicated researchers, patient educators, and physician trainers with the shared goal of meeting the need for high-end stem cell solutions and treatments.
Given that the group has a singular focus in this field, it is uniquely positioned to become the global leader in cellular medicine. In addition, by bringing together leading professionals in cellular medicine, it can discover issues that the industry faces and focus its research and development in this area. This specialization has, undoubtedly, enabled it to come up with solutions that address some of the significant issues that most stakeholders are facing in the industry.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
Global Stem Cells Group Announces Opening of New Research Facility at Marmara University’s Main Campus in Istanbul, Turkey
Collaboration Set to Propel Regenerative Medicine Research and Innovation
Miami, May 20, 2023 – Global Stem Cells Group, a leading provider of regenerative medicine solutions, is thrilled to announce the opening of its state-of-the-art research facility at Marmara University’s Main Campus in Istanbul, Turkey. This collaborative endeavor between Global Stem Cells Group, ReGen (described below), and Marmara University aims to advance research and knowledge in the fields of neurological conditions, autoimmune diseases, and the validation of safety and efficacy of procedures and Cellgenic products.
ReGen, a globally recognized network of clinics and a commercial partner of Global Stem Cells Group, is dedicated to providing cutting-edge cellular therapies that enhance patient well-being. This joint effort between ReGen, Global Stem Cells Group, and Marmara University seeks to drive research and innovation in cell therapy and tissue engineering.
Renowned for its commitment to excellence in education, research, and innovation, Marmara University provides an exceptional foundation for Global Stem Cells Group’s new research facility. With a strong emphasis on interdisciplinary collaboration and forefront research, Marmara University has emerged as a hub of intellectual discovery and academic achievement. The University’s renowned faculty and state-of-the-art infrastructure make it an ideal partner for Global Stem Cells Group in driving advancements in regenerative medicine.
By establishing this collaborative research facility at Marmara University, Global Stem Cells Group plans to access to the university’s wealth of expertise and resources, enabling the organization to undertake extensive clinical studies and groundbreaking research. Together, they will focus on addressing the challenges posed by neurodegenerative diseases and other medical conditions, aiming to develop innovative treatments and therapies that can significantly improve patient outcomes.
“Both Global Stem Cell Group and its commercial partner, ReGen, are thrilled to establish our new research facility in partnership with Marmara University.” Benito Novas, CEO of Global Stem Cells Group, expressed his enthusiasm for the collaboration. “This collaboration holds significant meaning for us, particularly in terms of the Cellgenic brand,” he added.
The Cellgenic product line encompasses a range of point-of-care regenerative medicine products, including autologous and allogeneic therapies, as well as exosomes. After examining the independent testing and validation of these products employed by Marmara University, Global Stem Cell Group is confident in the University’s quality and effectiveness.
The newly inaugurated research facility is expected to serve as a hub for extensive clinical studies on various medical conditions, with a particular focus on neurodegenerative diseases and ailments treatable through cell therapy and tissue engineering. This research is expected to contribute to the advancement of medical knowledge and the development of innovative treatments that have the potential to transform the lives of patients worldwide.
Global Stem Cells Group and its commercial partner, ReGen, extend their gratitude to Marmara University for its unwavering commitment to advancing regenerative medicine research and innovation. The collaboration between Global Stem Cells Group, ReGen, and Marmara University signifies what the parties believe is a significant milestone in the field of regenerative medicine, combining the expertise and resources of three leading institutions to drive breakthrough discoveries and shape the future of healthcare.
For more information about Global Stem Cells Group and its regenerative medicine solutions, please visit https://www.stemcellsgroup.com/.
About Global Stem Cells Group
Global Stem Cells Group is a leading provider of regenerative medicine solutions, dedicated to advancing healthcare through innovative therapies. With a global presence and a multidisciplinary team of experts, the organization strives to harness the potential of stem cells and regenerative medicine to improve patient outcomes across various medical fields. Through its extensive network of clinics, research facilities, and training centers, Global Stem Cells Group is committed to pushing the boundaries of regenerative medicine and making a positive impact on global healthcare.
About ReGen
ReGen is a renowned network of clinics and commercial partner of Global Stem Cells Group worldwide, offers cutting-edge cellular therapies to enhance the well-being of its patients. ReGen aims to drive research and innovation in cell therapy and tissue engineering together with Global Stem Cells Group and Marmara University.
About Marmara University
Marmara University is a prestigious educational institution renowned for its commitment to excellence in education, research, and innovation. With a strong emphasis on interdisciplinary collaboration and cutting-edge research, Marmara University has emerged as a hub of intellectual discovery and academic achievement. The university’s renowned faculty and state-of-the-art infrastructure make it an ideal partner for Global Stem Cells Group in driving advancements in regenerative medicine.
Safe Harbor Statement: Statements in this news release may be “forward-looking statements”. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions, or any other information relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates, and projections about our business based partly on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties, and assumptions that are difficult to predict. Therefore, actual outcomes and results may and are likely to differ materially from what is expressed or forecasted in forward-looking statements due to numerous factors. Any forward-looking statements speak only as of the date of this news release, and The Global Stem Cells Group undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date of this news release. This press release does not constitute a public offer of any securities for sale. Any securities offered privately will not be or have not been registered under the Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
- Published in Press Releases
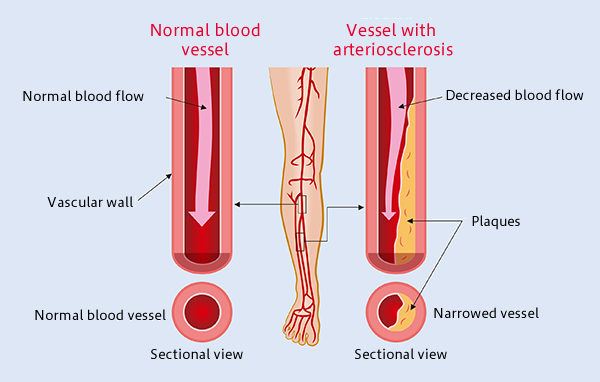
Atherosclerosis: The Most Common Form of Arterial Occlusive Disease in Adults
Introduction to Atherosclerosis
Atherosclerosis is the most common form of arterial occlusive disease in adults. About 15 percent of adults over 55 years of age suffer from critical ischemia, the most severe form of this disease.
Due to the gradual aging of the population and the growing number of people in their third age group, a number of studies have been conducted in order to improve the prognosis of atherosclerosis obliterans and to find alternatives to the mutilation of the extremities. As a general rule, chronic ischemia of the lower limbs should be treated to alleviate symptoms, particularly pain, prevent disease progression, and reduce the rate of amputations. In most patients with critical ischemia, the main goal is to preserve the affected limb.
Atherosclerosis Obliterans Grade IV
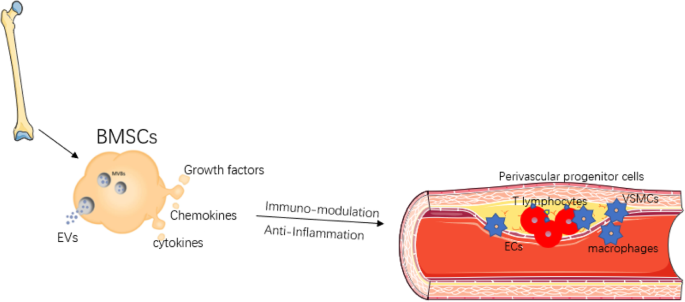
The development of regenerative medicine is closely linked to the development of new knowledge about embryonic and adult stem cells, as well as the regenerative and therapeutic potential of stem cell therapy. The use of adult stem cells in the treatment of peripheral artery diseases has been demonstrated as a therapeutic agent for inducing angiogenesis. Recent preclinical studies, as well as pioneering clinical studies, indicate that bone marrow-derived mononuclear cells (MBMCs) can enhance tissue vascularization in ischemic limbs, with results similar to those obtained with peripheral blood stem cells supply.
Case Studies and Research
Cuba presented the first studies carried out in 2004 at the Institute of Hematology of the “Enrique Cabrera” hospital in Havana City, which achieved encouraging clinical results and had very few adverse effects in recent years.
A progressive rise in the accumulated experience with stem cells was also observed in Pinar del Rio in 2005, as the first 10 cases were carried out. The rising ease of obtaining this type of cell has made research and applications with these cells advance rapidly with great expectations in terms of clinical application.
Research Findings on Atherosclerosis Obliterans Grade IV
A study published by Dia-Diaz, et al. in the Journal of Medical Sciences of Pinar del Rio examined 296 patients with grade IV atherosclerosis obliterans between 2009 and 2019. During the study, autologous stem cells were injected intramuscularly from peripheral blood. Within four weeks, pain relief was observed, as well as an increase in the pain-free claudication distance. Angiography after treatment revealed collateral vessel formation. The limb was saved in 201 patients (68%), while 95 cases (32%) presented amputation criteria. Complications were not reported following the procedure.
The study demonstrated the effectiveness of the implantation of autologous stem cells obtained from peripheral blood, as well as the favorable evolution of patients, clinical improvement of rest pain, walking distance without claudication, and ankle-brachial pressure index.
We still need to explore a lot of ground in terms of these and other conditions. You can learn more about regenerative medicine and stem cells by enrolling in our international certification program at www.issca.us.
- Published in Corporate News / Blog
Global Stem Cells Group announces the opening of a new Stem Cell Center in Bandung, Indonesia
Miami, FL, December 12, 2022 – Global Stem Cells Group (GSCG) is pleased to announce the opening of a new Stem Cell Center in Bandung, Indonesia in partnership with the Dr. Yanti Aesthetic Clinic. This joint venture will be the second in the country as they already have another facility in the stunning City of Surabaya.
The New Global Stem Cell Group Facility is Expected to Transform the Regenerative Medicine Field
The GSCG’s new center in Bandung aims to increase awareness about the benefits of regenerative medicine. Its launch strengthened the relationship between GSCG and the Dr. Yanti Aesthetic Clinic. This new facility is expected to transform regenerative medicine in the following ways:
- Offering accessible stem cell therapy to Indonesians;
- Promoting regenerative medicine technology; and
- Inspiring other doctors and industry experts to explore regenerative medicine.
Top Government Officials Attended the Launch of the Global Stem Cell Group Office in Bandung
The launch of the new Global Stem Cell Group facility in Bandung was a remarkable event in the medical field. The Minister of Tourism was among the top officials that witnessed the launch. Furthermore, the office of the Vice President sent several representatives.
What the President of Global Stem Cell Group Has to Say
Benito Novas, the president of the GSCG, was among the top industry specialists that graced the launch of the company’s second facility in Indonesia. According to him, GSCG wants to make regenerative medicine readily available for patients worldwide. Likewise, the company is encouraging more doctors to adopt stem cell therapy’s clinical and aesthetic applications in their work.
Benito Novas said: “We are dedicated to making it possible for both doctors and patients in all parts of the world to experience the benefits of regenerative medicines. The company is expanding and establishing itself as a market leader.”
The Director of the Dr. Yanti Aesthetic Clinic Appreciates GSCG
Dr. Yanti Kushmiran, director of the DR. Yanti Aesthetic Clinic, is once again honored to be part of this step into the future of regenerative medicine with its second Stem Cell Center in Indonesia together with the leading company Global Stem Cells Group.
Regarding this new clinic, Dr. Yanti says “We are honored to be part of GSCG, which has a more than 10-year track record in the market and a strong international reputation and to open a second cell therapy and regenerative medicine center facility under the Stem Cell Center brand. We will be able to provide more services to our patients as a result of this new partnership.”
Another GSCG Clinic is Opening in Indonesia by January 2023
The Global Stem Cell Group will continue increasing patients’ access to advanced regenerative medicine. As a result, the company plans to open another branch in Jakarta, the Capital of Indonesia. GSCG and its partners plan to launch this third facility in January 2023.
About Global Stem Cell Group
The Global Stem Cell Group is a family of several companies focused on stem cell medicine and research. The company uses its network to bring leadership in regenerative medicine training, research, and patient applications.
GSCG’s mission is to allow physicians to present the benefits of stem cell medicine to patients worldwide. The company also partners with policymakers, educators, and regulators to promote regenerative medicine.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website www.stemcellsgroup.com or call +1 305 560 5331
- Published in Press Releases
Global Stem Cells Group, Announces Launch of New Stem Cells and Regenerative Medicine Clinic in CDMX
Miami, FL, October 28, 2022 – Global Stem Cells Group announces a new partnership that enhances its goal of establishing its therapies and technology to meet market demand in populated areas of the world.
This collaboration with STEM LIFE clinic’s new facility and Dr. Vanessa Rodriguez Pares, currently one of the most prestigious aesthetic clinics in Mexico City, is expected to promote a high level of service in regenerative medicine throughout the country.
As part of this effort, the International Society for the Application of Stem Cells (ISSCA) has granted Dr. Vanessa Rodriguez Peers affiliation and use of their brand, products, therapies and training on how to apply stem cell therapies.
“This new partnership aims to expand the Global Stem Cells Group (GSCG) brand and create centers of excellence in cell therapy to meet the high demand in the Mexican market,” said Benito Novas, CEO of Global stem cells group “GSCG is rapidly expanding its global operations as it seeks to become a major player in the lucrative regenerative medicine industry. To achieve our expansion plans, our organization is partnering with healthcare providers specializing in regenerative medicine with at least five years of experience in the healthcare sector.”
Stem cell therapy is becoming an increasingly effective clinical solution for treating conditions that traditional or conventional medicine only offers within palliative care and pain management. Patients around the world are seeking a natural regenerative alternative without the potential risks and side effects sometimes associated with conventional pharmaceuticals.
The opening of this center, which will include the construction of an autologous tissue processing laboratory and an allogeneic tissue bank, will help stem cell therapy and regenerative medicine finally move from being an elective procedure to being accessible to patients throughout Mexico.
About Dr. Vanessa Rodriguez Pares
Dr. Vanessa Rodriguez Pares is a specialist in aesthetic medicine and surgery, with special attention to obesity and overweight in all ages. Management of cosmetic surgery with a comprehensive approach to the patient, taking into account their safety and their physical, psychological and emotional needs. Specialist in ULTHERAPY treatment to perform facelift without surgery. In addition to extensive experience with cellular therapies and regenerative medicine since 2016, specializing in anti-aging techniques, hair regeneration, facial aesthetics with stem cell assisted lipotransfer among other techniques.
About the Global Stem Cell Group
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products, and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators, and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website
www.stemcellsgroup.com or call +1 305 560 5331
- Published in Press Releases
GSCG Announces the Appointment of Dr. Rafael Moguel as New Chief Medical Officer (CMO) of Cellular Hope Institute Cancun
Global Stem Cells Group (GSCG) has announced the appointment of Dr Rafael Moguel as the new Chief Medical Officer (CMO) of Cellular Hope Institute Cancun.
Dr. Moguel, Fellow of the Society for Cardiac Angiography and Intervention (SCAI), Member of the Mexican Society for Interventional Cardiologists, and Member of the Latin American Society for Interventional Cardiology, comes with a wealth of experience and training.
As CMO with vast knowledge and experience, Dr Moguel is expected to change the direction of Cellular Hope Institute Cancun, which offers patients the best treatments in multiple areas, such as sports medicine, pediatric care, chronic degenerative disease, and autoimmunity. It’s a multi-specialty training center for cellular therapies and regenerative medicine, offering the most advanced treatments in cell therapy.
“I have full confidence in the appointment of Dr Rafael Moguel as the CMO of Cellular Hope Institute Cancun,” said Benito Novas, CEO of Global Stem Cells Group. The CEO also expressed his satisfaction in Dr Moguel’s abilities and experience, and is confident that (Dr Moguel) will advance the objectives of Cellular Hope Institute and propel it to new heights.
With his impressive resume, Dr. Moguel has served in various capacities across several top medical institutions. He was the assistant professor for Cardiology and Interventional Cardiology at Hospital 1° de Octubre, where he also handled hemodynamics and internal medicine.
In his acceptance speech, Dr Moguel said, “I want to affirm and reaffirm my commitment to the goals of Cellular Hope Institute.” With his elaborate working experience and professionalism in cellular therapy, he expressed his confidence in rising to the expectations of Cellular Hope Institute.
Dr. Moguel received his medical degree at Universidad Veracruzana in 1983. He holds a valid certification by the Mexican Cardiology Board for Interventional Cardiology. With more than 10,000 interventional procedures, most notably peripheral and brain intervention, coronary, pacemakers and vein interventions, we believe Dr. Moguel has all it takes to propel Cellular Hope Institute to the next level.
The appointment of Dr. Moguel comes barely two months after an earlier announcement by GSCG about opening the multispecialty regenerative medicine center in Cancun. The facility’s main objective is to incorporate different treatments basing on regenerative medicine, and serve as a training center for cellular therapies and regenerative medicine.
We believe Dr. Moguel’s rich background as an assistant professor and head of interventional cardiology and hemodynamics positions him as the best candidate to run Cellular Hope Institute. His experience is expected to make this facility one of the best cellular therapy centers worldwide, and provide more specialized treatment for patients requiring regenerative medicine.
About Cellular Hope Institute
This facility is a center for both patients and medical experts worldwide, and its main objective is to benefit medical experts and patients globally. The facility achieves this objective through offering the best treatments for patients with multiple conditions, such as spinal cord injury, chronic obstructive pulmonary, sports medicine, and autoimmunity, among others. If you need more information about Cellular Hope Institute, please contact us today.
About the Global Stem Cell Group
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products, and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators, and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website.
- Published in Press Releases