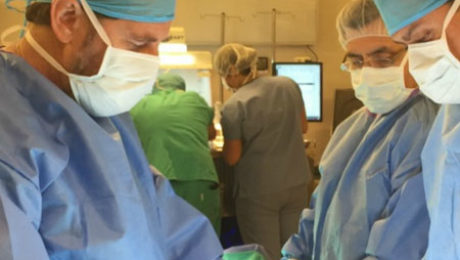
Global Stem Cells Announces Formal Inaugural for Clinica Biomaster in Costa Rica
Global Stem Cells Group has launched Clinica Biomaster Costa Rica with an inauguration and symposium at Clinica Biomaster, the company’s new stem cell center in Escazú, Costa Rica.
Global Stem Cells Group hosted a symposium and formal inauguration of Clinica Biomaster Costa Rica, the company’s new stem cell center in Escazú, Costa Rica. Joseph Purita, M.D., head of the Global Stem Cells Group Scientific Advisory Board, was the keynote speaker at the event, held July 15 and 16, 2016.
Purita also presented lectures to medical staff at Hospital CIMA and Hospital Metropolitano in San Jose during the inaugural weekend.

Joseph Purita, M.D.
The two-day symposium officially launched Global Stem Cells Group’s Costa Rica operations, which includes plans for four stem cell training courses for physicians and a regenerative medicine symposium in early 2017.
Clinica Biomaster is headed by neurologist and anti-aging specialist Dra. Mariella Tanzi, founder of BIOMEN S.A. Tanzi and Biomaster have formed an alliance with GSCG to be the exclusive representative for the Miami-based biomedical company’s products and services in the Costa Rica market.
The symposium included sessions on clinical advances in stem cell research; molecular biology; models of treatment in surgical and cosmetic applications, and in clinical conditions; application of minimally manipulated stem cells in the physician’s office; stem cells, regenerative medicine and its application in anti-aging medicine and medical legal issues. It also included a full day, hands-on training session to provide participating physicians and qualified medical professionals with state-of-the-art techniques for isolating and re-integrating adipose- and bone marrow-derived stem cells for in office patient treatments, along with clinical protocols.
 This popular training course is part of the Global Stem Cells Group’s commitment to the growing network of world-class stem cell researchers, treatment practitioners and investors committed to advancing stem cell medicine, and helping physicians bring treatments into the office for the benefit of patients.
This popular training course is part of the Global Stem Cells Group’s commitment to the growing network of world-class stem cell researchers, treatment practitioners and investors committed to advancing stem cell medicine, and helping physicians bring treatments into the office for the benefit of patients.
To learn more, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas(at)regenestem(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
To review this press release live online, click here
###
- Published in Press Releases
New Guidelines for Stem Cell Research and Therapies Aim to Protect Patients from Charlatan Quackery
Introduction
Stem cell research has advanced significantly, leading to a myriad of treatment options. However, the field faces challenges from unscrupulous providers and premature publicity.
Professional Guidelines for Responsible Stem Cell Research
International Society for Stem Cell Research (ISSCR)

The ISSCR leads in setting high standards for translational stem cell research:
- Emphasizes rigorous preclinical evidence and peer review.
- Highlights the importance of IRB review and comprehensive informed consent.
International Society for Cellular Therapy (ISCT)
The ISCT expands its scope beyond stem cells, advocating for broader cell-based interventions:
- Focuses on defining scientific evidence and regulatory practices.
- Addresses implications across clinical practice and commercialization.
Development of New Guidelines
Terminology and Scientific Evidence
Efforts are underway to standardize terminology and define scientific evidence levels critical for ethical and effective stem cell therapy.
ISSCR’s 2016 Guidelines Update
In 2016, ISSCR updated guidelines cover:
- Emerging technologies like gene editing and induced pluripotent stem cells (iPSCs).
- Upholding ethical standards such as the “14-day rule” for embryo experimentation.
Key Topics Addressed in the Revised Guidelines
The updated guidelines include:
- Oversight processes for embryo research and mitochondrial replacement therapy.
- Standards for preclinical and clinical research, emphasizing safety and efficacy.

Advancements in Stem Cell Research
Stem cell therapies show promise in treating a range of conditions, leveraging pluripotent stem cells for tissue repair and genetic disease treatments.
Conclusion
Stem cell research continues to evolve responsibly, offering hope for future medical advancements while safeguarding patient interests against fraudulent practices.
- Published in Corporate News / Blog
Researchers Move Closer to Lung Stem Cell Therapies to Treat Chronic Lung Diseases
Introduction
Chronic lung diseases, including COPD, bronchitis, emphysema, and asthma, are significant causes of mortality in the U.S., highlighting the urgent need for advanced treatments.
Stem Cells in the Lung
Types and Functions of Lung Stem Cells
Human lungs are complex organs comprising:
- Conducting Airway Tubes: Includes trachea, bronchi, and bronchioles.
- Gas Exchange Regions: Alveolar spaces crucial for oxygen exchange.
Role of Progenitor Cells
Progenitor cells like tracheal basal cells and alveolar type 2 cells play a vital role in maintaining lung health by replacing old or damaged cells.
Diversity of Lung Stem Cells
Embryonic and adult lung stem cells contribute differently:
- Research indicates their role in lung development and regeneration, with potential implications for disease treatment.
Current Research on Lung Stem Cell Therapies
Adult Mesenchymal Stem Cells (hMSCs)
hMSCs are pivotal in:
- Immunomodulation: Regulating immune functions and secreting bioactive molecules for anti-inflammatory and regenerative effects.
- Versatility: Capable of generating various cell types, potentially aiding in lung tissue repair.
Insights into Lung Disease Causes
Understanding lung stem cell biology enhances knowledge of disease mechanisms like COPD, paving the way for innovative treatments.

Future Prospects of Lung Stem Cell Therapies
Translational Research and Clinical Applications
Ongoing studies aim to:
- Identify and characterize lung stem cells in humans, advancing potential clinical applications.
- Explore personalized medicine approaches using lung stem cells for targeted therapies.
Conclusion
Progress in lung stem cell research holds promise for developing effective therapies to combat chronic lung diseases, offering hope for improved patient outcomes and quality of life.
- Published in Corporate News / Blog
The History of Research on Adult Stem Cells: We’ve Come a Long Way
Introduction to Adult Stem Cells
Adult stem cells, also known as somatic stem cells, are undifferentiated cells found among differentiated cells in tissues or organs. They play a crucial role in tissue maintenance and repair.
Types and Functions of Adult Stem Cells
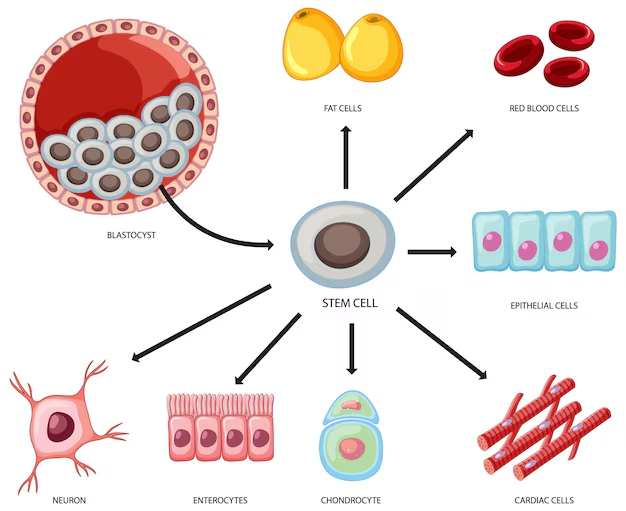
Bone Marrow Stem Cells
- Hematopoietic Stem Cells: Responsible for forming all blood cell types.
- Mesenchymal Stem Cells: Can differentiate into bone, cartilage, fat cells, and support blood formation.
Brain and Heart Stem Cells
Recent discoveries have identified stem cells in unexpected locations like the brain and heart, challenging previous beliefs and expanding potential applications in transplantation therapies.
Historical Development of Adult Stem Cell Research
Early Discoveries
In the 1950s, researchers identified hematopoietic stem cells in bone marrow, marking the beginning of significant research into adult stem cells.
Advances Over Decades
- 1960s: Discovery of neural stem cells in the brain.
- 1990s: Confirmation of adult brain’s capability to generate neurons and other cell types.
Distribution of Adult Stem Cells in the Body

Adult stem cells are found in various organs and tissues, residing in specific niches critical for their function and activation in response to tissue repair needs or disease.
Methods to Identify Adult Stem Cells
Scientists employ several techniques:
- Labeling and Differentiation: Using molecular markers to track specialized cell types generated.
- Transplantation Studies: Removing and labeling cells, then transplanting them to observe tissue repopulation.
Future Directions in Adult Stem Cell Research
Ongoing research aims to:
- Further characterize and manipulate adult stem cells for therapeutic purposes.
- Explore personalized medicine applications using stem cell technologies.
Conclusion
The study of adult stem cells has evolved significantly, offering promising avenues for understanding and treating various diseases. Continued research holds potential for transformative advancements in regenerative medicine.
- Published in Corporate News / Blog
How Clinical Trials on Stem Cell Therapies Work, and Where to Find Them
Introduction to Stem Cell Clinical Trials
Stem cell clinical trials play a crucial role in evaluating the safety and efficacy of new treatments. Accessing information about these trials is facilitated through dedicated registries like ClinicalTrials.gov and the WHO International Clinical Trial Registry Platform.
Key Resources for Stem Cell Clinical Trials
ClinicalTrials.gov
- Purpose and History: Established under the FDA Modernization Act to provide comprehensive information on clinical studies.
- Accessibility: Offers updated information directly from study sponsors or principal investigators.
WHO International Clinical Trial Registry Platform
- Supplementary Information: Provides additional details complementing ClinicalTrials.gov, enhancing accessibility and usability for researchers and participants.
Geographic Distribution and Focus Areas of Stem Cell Trials
- Location Trends: Predominantly conducted in the U.S. followed by Europe, as evidenced by data from ClinicalTrials.gov.
Phases of Clinical Trials
Global Distribution
Overview of Trial Phases
- Phase 0 to Phase IV: Sequential stages from initial safety assessments to post-marketing studies, each serving distinct purposes in drug development and evaluation.
Types of Stem Cell Clinical Trials

Interventional Studies
- Purpose: Volunteers are assigned interventions to evaluate biomedical or health outcomes based on specific protocols.
- Inclusion of Observational Studies: Descriptions of observational studies and expanded access programs for investigational drugs are also included.
Funding Sources for Stem Cell Trials
Analysis of Funding
- Primary Funders: Includes the National Institutes of Health (NIH), other federal agencies, industry partners, and diverse entities such as universities and community-based organizations.
- Impact of Funding: Highlights the diversity of financial support influencing stem cell research outcomes and accessibility.
Conclusion
Understanding the structure and resources of stem cell clinical trials is crucial for both researchers and participants seeking innovative treatments. Access to comprehensive trial information supports informed decision-making and advances in medical research.
- Published in Corporate News / Blog
Stem Cell Research and Stem Cell Therapy: When can stem cells be used to treat patients?
Understanding Stem Cell Research
Stem cells possess the unique ability to differentiate into various specialized cell types in the body, making them invaluable for regenerative medicine. They play a crucial role in replenishing damaged tissues throughout an individual’s life.
Types of Stem Cells in Research
- Embryonic Stem Cells: Derived from the inner cells of a blastocyst, these cells have the potential to form any type of cell in the body.
- Adult Stem Cells: Found in various tissues, these cells contribute to tissue repair and maintenance.
- Induced Pluripotent Stem Cells (iPSCs): Reprogrammed from adult cells to exhibit embryonic-like properties, offering new avenues for research and therapy.
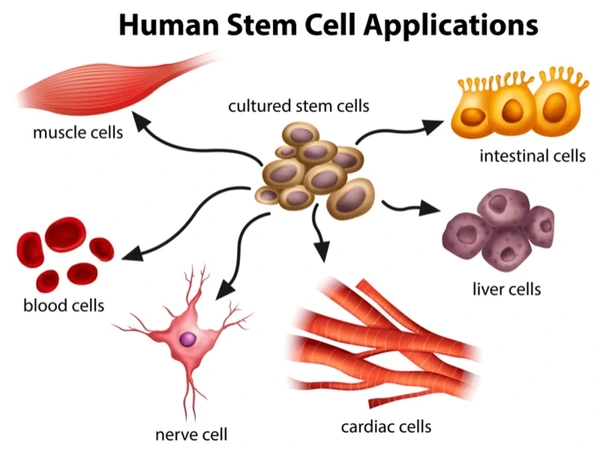
Applications of Stem Cells in Disease Treatment
Stem cell research holds promise for treating a wide array of diseases, including diabetes and heart disease, by harnessing their regenerative capabilities.
Challenges and Research Advances
- Laboratory Studies: Used to understand fundamental properties and differentiation mechanisms of stem cells.
- Drug Screening: Stem cells serve as models to test new drugs and study normal growth processes and disease mechanisms.
Ethical Considerations and Guidelines
The Declaration of Helsinki guides ethical practices in stem cell research involving human subjects, emphasizing informed consent and the need for ongoing evaluation of experimental interventions.
Clinical Translation of Stem Cell Therapies
- Regulatory Framework: Clinical trials are essential to evaluate the safety and efficacy of stem cell-based treatments.
- Phases of Clinical Trials: From initial safety assessments (Phase 0) to post-marketing studies (Phase IV), each phase plays a crucial role in determining treatment viability.
Future Directions in Stem Cell Research
Continued advancements in stem cell research are expected to expand our understanding of cellular regeneration and pave the way for innovative therapies.
- Published in Corporate News / Blog
Global Stem Cells Group Launches Two Stem Cell Treatment Centers in Arica and Iquique, Chile

Global Stem Cells Group announces the launch of two new stem cell treatment clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of the international biotech company’s expanding presence in Latin America.
MIAMI, May 31, 2016—Global Stem Cells Group, a leading international biotechnology company, announces the launch of operations at two new GSCG clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of GSCG’s expanding presence in Latin America.
Both the Arica and Iquique clinics offer the most advanced protocols and techniques in stem cell medicine to patients from around the world.

The clinics are headed by stem cell specialists Victor Perez, M.D., and Duval Aguirre, M.D., and will offer treatments in chronic degenerative conditions, Type 2 diabetes, COPD, traumatology and sports medicine.
Global Stem Cells Group, has been expanding its clinical presence throughout Latin America and worldwide by partnering with qualified physicians experienced in stem cell therapies to open new clinics. The new Arica and Iquique clinics are certified for the medical tourism market.
Global Stem Cells Group is committed to the highest standards in service and technology, expert and compassionate care, and a philosophy of exceeding the expectations of their international patients.
For more information, visit the Global Stem Cells Group website, Email bnovas@stemcellsgroup.com, or call 305-560-5337.
About the Global Stem Cells Group:

Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Gordie Howe’s Stem Cells Treatments Support a Growing Appeal for These Therapies Among Athletes and Baby Boomers
The Story of Gordie Howe’s Stem Cell Treatment
In October 2014, Gordie Howe, the legendary hockey player, faced a life-threatening stroke that left him severely debilitated. Despite medical efforts, including an experimental stem cell treatment in Mexico, his condition continued to deteriorate. However, after receiving neural and mesenchymal stem cells, Howe showed remarkable signs of improvement, including regaining mobility and cognitive function.
The Impact of Stem Cell Therapy on Gordie Howe’s Recovery
- Experimental Treatment: Howe’s treatment involved injecting neural and mesenchymal stem cells into his spinal canal, aiming for brain repair and anti-inflammatory benefits.
- Recovery Milestones: Within hours of the procedure, Howe exhibited unexpected improvements, such as walking unaided for the first time since his stroke.
Stem Cell Therapy: Appeal Among Athletes and Baby Boomers
Athletes’ Interest in Stem Cell Therapy
Athletes, prone to injuries and degenerative conditions, increasingly turn to stem cell therapy for its potential regenerative properties. Icons like Bart Starr and John Brodie have also pursued such treatments.
Growing Popularity Among Baby Boomers
The aging population, particularly baby boomers, seeks stem cell therapies to address age-related ailments such as joint degeneration and chronic conditions.
Controversies and Regulatory Challenges

Despite its appeal, stem cell therapy faces scrutiny due to regulatory issues and varying international standards. The FDA is developing guidelines to regulate these treatments, distinguishing between approved clinical trials and unauthorized clinics.
Regulatory Landscape and FDA Guidelines
- Current FDA Oversight: Most stem cell therapies require FDA approval, with exceptions for minimally manipulated cells.
- Unauthorized Clinics: Concerns persist over unauthorized stem cell clinics operating outside regulatory frameworks.
Future Directions and Considerations
As research and public interest in stem cell therapy continue to grow, ongoing debates over efficacy, safety, and regulatory oversight shape its future in medical practice.
- Published in Corporate News / Blog
Global Stem Cells Group Affiliate University of Santiago Biotechnology Lab Applies for RTA Certification
MIAMI, April 30, 2016–Global Stem Cells Group CEO Benito Novas announced that the University of Santiago has become the latest GSCG affiliate to apply for Regenerative Technologies Alliance (RTA) certification. RTA certification represents the highest standards of excellence in current medical and laboratory practices against established standards.
The announcement comes less than a week after Global Stem Cells Group announced a mutual endorsement for an Asian-Pacific alliance with the university to host a regenerative medicine and stem cell symposium July 1-2 at the  university’s Santiago campus. Through the alliance, the two organizations have established a working agenda for collaborative initiatives and educational programs in stem cell and regenerative medicine research and development for 2016 – 2020.
university’s Santiago campus. Through the alliance, the two organizations have established a working agenda for collaborative initiatives and educational programs in stem cell and regenerative medicine research and development for 2016 – 2020.
Experts in the field of regenerative and cell-based medicine conduct the RTA certification program. The Regenerative Technologies Alliance, a 501(c)3 non-profit organization operated by Regenetech and supported by individuals and institutions committed to bringing peer oversight and transparency to the regenerative and cell-based medicine industry, provides certification for:
- Laboratory Facilities that provide the processing of human tissue for the development of cell-based applications
- Clinical facilities seeking to participate in authorized studies that are approved by Institutional Review Boards (IRB)
- Medical facilities providing cell-based medical therapies to patients.
 According to Benito Novas, CEO of Global Stem Cells Group, the certification process will be conducted under the direction of David B. Audley, RTA Chair and General Secretary. Audley is a highly regarded medical marketing and management services consultant who co-founded MedTourGlobal after 10 years in the emerging field of regenerative medicine.
According to Benito Novas, CEO of Global Stem Cells Group, the certification process will be conducted under the direction of David B. Audley, RTA Chair and General Secretary. Audley is a highly regarded medical marketing and management services consultant who co-founded MedTourGlobal after 10 years in the emerging field of regenerative medicine.
Santiago was the city chosen for the first RTA accreditation in Latin America. This type of accreditation differs from traditional accreditation processes in that it examines all aspects of service and patient care, considers the progress of the location and the potential for receiving international patients, as well as meeting quality expectations.
The alliance between Global Stem Cells Group and the University of Santiago extended an invitation to Audley and started the RTA application and review process to assess the reception, harvesting, bio-processing and application of stem cells, in the university’s Biotechnology and Molecular Biology Laboratory and Providencia Stem Cells Center. This international certification opportunity includes evaluating and reinforcing physicians working in those areas, and validating the processes and bio-technological resources against international standards.
RTA delegates are expected to visit the University of Santiago facility in July to conduct a physical inspection of the premises. The goal is to create a cell therapy hub in Santiago that can accommodate the needs of patients in neighboring countries seeking stem cell and regenerative medicine treatments. Strong patient demand in the United States and Europe have also created a growing interest in the medical tourism industry.
Global Stem Cells Group Chief Medical Officer Enrique Testart, M.D., heads a medical and scientific team tasked with establishing a new edition of the post-graduate diploma program, “Diplomat in Cell Therapy and Tissue Engineering” at Santiago University.
The theoretical and practical, 120-hour program will deliver the fundamentals of the most advanced cell therapies and clinical applications currently practiced safely and effectively in more than 35 cities worldwide.
Testart and Novas have collaborated on the push to highlight Chile’s appeal as a destination for patients seeking stem cell therapies for a variety of conditions, and as part of GSCG’s ongoing commitment to expand the reach of stem cell treatments throughout Latin America. Global Stem Cells Group’s Quito, Chile clinical facility is also in the process of earning RTA certification.
Novas says that GSCG aims to have all the company’s clinical facilities earn RTA certification by the end of 2016.
Global Stem Cells Group’s presence in Chile represents one of the largest integrated science and technology organizations associated with tissue engineering and tissue regeneration. GSCG represents the areas of research, development, medical appliances, equipment and protocols for clinical standards, and is fast becoming a global leader in adult stem cell therapies, medical training in stem cell protocols, and the manufacture of high quality products and supplies in regenerative medicine.
Their strategic leadership puts them in the forefront of stem cell research, medical education and patient care. Global Stem Cells Group comprises six companies, each of which works in specialty areas related to the stem cell industry .
To learn more, visit the Global Stem Cells Group website, Email bnovas(at)stemcellsgroup(dot)com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
###
To view this press release live online, click here
- Published in Press Releases
Global Stem Cells Group Announces Cell Assisted Fat Transfer Training
MIAMI, April 30, 2016–Global Stem Cells Group and Stem Cell Training, Inc. have announced the addition of a cell assisted fat transfer training course, to be conducted by Alfredo Hoyos, M.D., head of the GSCG Advisory Board, and Enrique Testart, M.D., GSCG Chief Medical Officer.
Two courses are scheduled, one to be conducted in Santiago, Chile August 23-24, and the other in Cancun, Mexico October 11-12.
The training course involves facial fat transfer injection combined with adipose-derived stem cells, to address the primary issues that concern patients in the aesthetic field, including facial aging caused by volume loss.
Hoyos is a plastic surgeon and stem cell expert who founded Stemlab, a Bogota, Colombia facility that conducts extensive research in regenerative medicine in an effort to establish stem cell treatments that can repair damaged tissue in living organisms.

Alfredo Hoyos, MD
Stemlab works to further the development of aesthetic techniques using stromal stem cells derived from adipose tissue. Hoyos is the inventor of high definition liposculpture (HDL) and dynamic definition lipoplasty (4D) techniques as well as other advanced techniques that focus on body contouring.
Testart is a surgeon specializing in child trauma microsurgery. He is also a medical entrepreneur and founder of Consortia Innovas S.A. in Santiago, Chile, an organization dedicated to consulting and clinical health management for clinical management firms and research and development-oriented planners on the latest treatments in regenerative medicine as they become available.
Enrique Testart, MD
Hoyos and Testart will train qualified physicians on cell assisted fat transfer techniques and protocols, during which fat cells are harvested from the patient’s own body and redistributed to other areas of the body to augment sunken or thin regions of the face or body in order to add volume where it is desired.
Lipoinjection for cosmetic treatments can be unpredictable, and has a low rate of graft survival due to partial necrosis. To overcome these problems, cell assisted fat transfer (lipostransfer) was developed as a strategy wherein autologous adipose-derived stem (stromal) cells (ASCs) are used in combination with lipoinjection. A stromal vascular fraction (SVF) containing ASCs is freshly isolated from half of the aspirated fat and recombined with the other half. This process converts relatively ASC-poor aspirated fat to ASC-rich fat, reducing postoperative atrophy of injected fat to a minimal leve l that clinical trials have found does not change substantially after 2 months. Patients walk away with soft and natural-appearing augmentation.
l that clinical trials have found does not change substantially after 2 months. Patients walk away with soft and natural-appearing augmentation.
Cell assisted fat transfer is a promising treatment for facial rejuvenation and soft tissue augmentation because there are no incisional scars or complications associated with foreign materials.
The cell assisted fat transfer training course will be offered through Global Stem Cells Group affiliate Stem Cell
To learn more, visit the Global Stem Cells Group website, or the Stem Cell Training website, email 
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
###
To view this press release live online, click here
- Published in Press Releases