Understanding Stem Cell Research
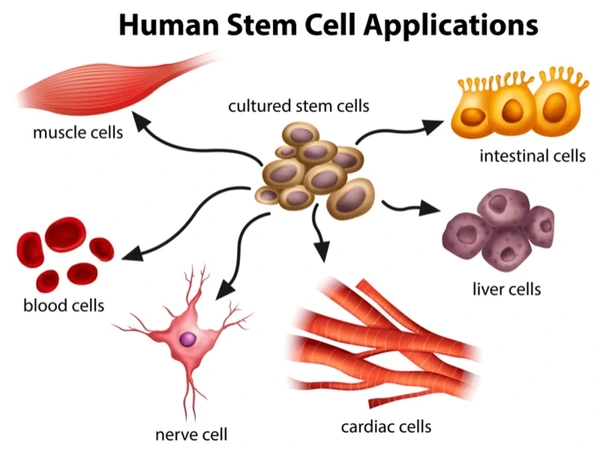
Stem cells possess the unique ability to differentiate into various specialized cell types in the body, making them invaluable for regenerative medicine. They play a crucial role in replenishing damaged tissues throughout an individual’s life.
Types of Stem Cells in Research
- Embryonic Stem Cells: Derived from the inner cells of a blastocyst, these cells have the potential to form any type of cell in the body.
- Adult Stem Cells: Found in various tissues, these cells contribute to tissue repair and maintenance.
- Induced Pluripotent Stem Cells (iPSCs): Reprogrammed from adult cells to exhibit embryonic-like properties, offering new avenues for research and therapy.
Applications of Stem Cells in Disease Treatment
Stem cell research holds promise for treating a wide array of diseases, including diabetes and heart disease, by harnessing their regenerative capabilities.
Challenges and Research Advances
- Laboratory Studies: Used to understand fundamental properties and differentiation mechanisms of stem cells.
- Drug Screening: Stem cells serve as models to test new drugs and study normal growth processes and disease mechanisms.
Ethical Considerations and Guidelines
The Declaration of Helsinki guides ethical practices in stem cell research involving human subjects, emphasizing informed consent and the need for ongoing evaluation of experimental interventions.
Clinical Translation of Stem Cell Therapies
- Regulatory Framework: Clinical trials are essential to evaluate the safety and efficacy of stem cell-based treatments.
- Phases of Clinical Trials: From initial safety assessments (Phase 0) to post-marketing studies (Phase IV), each phase plays a crucial role in determining treatment viability.
Future Directions in Stem Cell Research
Continued advancements in stem cell research are expected to expand our understanding of cellular regeneration and pave the way for innovative therapies.