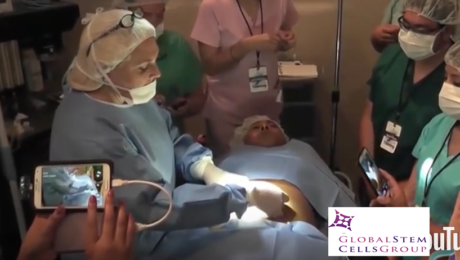
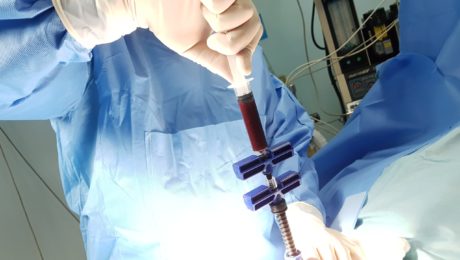
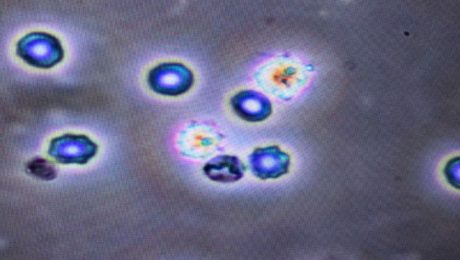
MIAMI, Feb 19, 2018—Leslie Mesen, M.D., a leader in the field of anti-aging medicine in Latin America and medical director of Global Stem Cells Group affiliate Stem Cell Center in Escazu, Costa Rica, will be a keynote speaker at the International Association for Stem Cell Application (ISSCA) Regenerative Medicine Cell Therapies Symposium in Istanbul, Turkey April 28, 2018.

Leslie Mesen, M.D.
Three months post-injury, the patient’s foot wound was not healing. Mesen administered an SVF autologous stem cell procedure and, four-weeks post-stem cell treatment, the wound showed significant improvement (70 percent wound closure) with clear new tissue growth visible around the wound. Mesen then performed an activated PRP procedure to further stimulate cell division.
Two weeks after the PRP treatment, the patient was walking in shoes, and underwent a final stem cell procedure. Over a period of five weeks, the patient—an electrician– recovered the ability to care for himself. He is expected to return to work soon.
Mesen is board certified in anti-aging and regenerative medicine by The American Academy of Anti-Aging Medicine and a pioneer in anti-aging medicine in Latin America.
He has extensive experience as the chief medical officer of three highly recognized anti-aging institutes: Costagenics Age Management program (2007-2010), Anti-Aging Institute of the Americas (2010-2013) and the Anti-Aging and Wellness Clinic (2014- present).
Mesen studied medicine at Universidad Iberoamericana (UNIBE) in San José, Costa Rica and receives continuing education courses in the United States and Latin America. In the U.S., he has trained at the University of Miami Jackson Memorial Hospital. His range of services encompass all aspects of aging, delivering programs tailored to each patient’s unique needs. Mesen offers platelet-rich plasma (PRP) therapy, HRT, hair regrowth treatments, intravenous infusions, and a range of other anti-aging and aesthetic treatments.
The Istanbul international symposium is part of ISSCA’s mission to support a paradigm shift from traditional healthcare solutions to regenerative medicine and provide the latest innovative discoveries and developments in all areas of stem cell research. The symposium will host a group of renowned international speakers, experts in the field of stem cell and regenerative medicine, who will provide a full day of rigorous scientific discourse directed to physicians.
The day’s events will incorporate information on stem cell biology, medicine, applications, regulations, product development, and commercialization, business opportunities, challenges, and potential strategies for overcoming those challenges.
To participate in the ISSCA Istanbul Symposium, reserve your spot by registering today.
For more information, visit the stemcellconference.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About International Society for Stem Cell Application (ISSCA):
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine
and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
###
ISSCA Leslie Mesen
- Published in Press Releases
How does the U.S. FDA regulate cell therapies?
Cell therapies in the United States are regulated by the FDA’s Office of Cellular, Tissue, and Gene Therapies (OCTGT), part of the FDA Center for Biologics Evaluation and Research (CBER). Understanding these regulations is crucial for companies involved in the development and distribution of cellular products.
Overview of FDA Regulation
The FDA’s Center for Biologics Evaluation and Research (CBER) oversees:
- Cellular therapy products
- Human gene therapy products
- Certain devices related to cell and gene therapy
CBER operates under the authority of the Public Health Service Act and the Federal Food Drug and Cosmetic Act, which provide the legal framework for regulatory oversight.
Regulation Pathways: 351 vs. 361 Products
361 Products
Products that meet all criteria outlined in 21 CFR 1271.10(a) are classified as Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) under Section 361 of the Public Health Service (PHS) Act. These products, known as “361 products,” do not require FDA licensure or approval.
351 Products
Products that do not meet all criteria outlined in 21 CFR 1271.10(a) are regulated under Section 351 of the PHS Act and the Federal Food, Drug, and Cosmetic Act. These products are treated as drugs, devices, or biological products and require FDA approval through clinical trials to demonstrate safety and efficacy.
Regulatory Framework
The distinction between 361 and 351 products reflects the FDA’s assessment of relative risk associated with each type of cell therapy. 361 products are considered lower risk and are subject to less stringent regulatory requirements compared to 351 products, which undergo a rigorous approval process akin to pharmaceuticals.
Conclusion
Navigating FDA regulations for cell therapies involves understanding whether a product falls under the 361 or 51 pathway. Compliance with these regulations is essential for ensuring safety, efficacy, and legal market entry for cell therapy products in the United States.

- Published in Corporate News / Blog
ISSCA Announces Regenerative Medicine Cell Therapies Symposium in Istanbul, Turkey April 28, 2018
(Image: Istanbul, Turkey)
The International Association for Stem Cell Application (ISSCA) has announced plans to host a regenerative medicine symposium in Istanbul, Turkey April 28, 2018.
MIAMI, Feb 14, 2018—The International Association for Stem Cell Application (ISSCA) has announced plans to host a regenerative medicine symposium in Istanbul, Turkey April 28, 2018. The symposium will focus on new advances in cell therapies.
The Istanbul international symposium is part of ISSCA’s mission to support a shift from traditional healthcare solutions to regenerative medicine and provide the latest innovative discoveries and developments in all areas of stem cell research. The symposium will host a group of renowned international speakers, experts in the field of stem cell and regenerative medicine, who will provide a full day of rigorous scientific discourse directed to physicians.
The day’s events will incorporate information on stem cell biology, medicine, applications, regulations, product development, and commercialization, business opportunities, challenges, and potential strategies for overcoming those challenges.
To learn more about the ISSCA symposium in Istanbul and to register to attend, visit the stemcellconference.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About International Society for Stem Cells Applications
 The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
Regenerative Medicine symposium Istanbul
- Published in Press Releases
Global Stem Cells Group to Launch Regenerative Medicine Training Program for Mexico at Congreso Internacional de Medicina Antienvejecimiento y Longevidad
(Image: Regenerative Medicine Training Program for Mexico)
MIAMI, Feb. 14, 2018— Global Stem Cells Group, a world leader in stem cell and regenerative medicine, will launch the organization’s complete Regenerative Medicine Training Program for Mexico at the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad (Congress in Anti-Aging and Longevity Medicine) to be held February 16 – 18, 2018 in Mexico City.
The training course will be held in Mexico City April 13 – 14, 2018.
The “Practical Course of Cell Therapies in a Clinical Environment” is a two-day, hands-on training course that provides physician attendees with the intellectual property of 22 proprietary protocols that allow treatment procedures for degenerative and aesthetic conditions and diseases in-office, as well as step-by-step videos of each protocol.
Course attendees will acquire the skills that will allow them to offer alternative therapies to patients who suffer from degenerative conditions for which no traditional medical solutions are available. Attendees will treat three to five live patients during the course. Upon completing the course successfully, participants will join a select group of physicians at the forefront of medical science—only 5 percent of physicians worldwide have access to studies of cell therapies, and only 0.01 percent are currently practicing these therapies.
Qualified physicians learn skills that can be used to treat patients in-practice, and utilize the training for career advancement.
Participating physicians will also receive access to the online stem cell training course to review all content and procedures introduced in the 2-day clinical training course, patient forms and guidelines, procedure informed consent forms, didactic lectures, training booklets, and more.
The Regenerative Medicine Training Program for Mexico was developed for physicians and high-level practitioners to learn techniques in harvesting and reintegrating stem cells. Stem cell therapies continue to revolutionize the healthcare industry and help improve the quality of life for patients.
To learn more about the certification training course and register to participate, visit the Stem Cell Training Course website, email info@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group
 Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Regenerative Medicine Training for Mexico
- Published in Press Releases
Global Stem Cells Group CEO Benito Novas to be a Keynote Speaker at Congreso Internacional de Medicina Antienvejecimiento y Longevidad Feb. 16-18, 2018
Benito Novas, Global Stem Cells Group CEO
MIAMI, Feb. 14, 2018— Global Stem Cells Group founder and CEO Benito Novas will be a keynote speaker at the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad (Congress in Anti-Aging and Longevity Medicine) February 16 – 18, 2018 in Mexico City. The conference is organized by The Institute of Anti-Aging and Longevity Medicine, the leading anti-aging and regenerative medicine society in Mexico.
Novas, a global entrepreneur and medical marketing professional in the fields of biotechnology, life sciences, and healthcare development, will share his expertise on the latest marketing tools for managing a medical practice. Topics will include social media strategies and tools for promoting physician practices, and how to design Google and Facebook ads that generate targeted leads and engage new potential patients.
The healthcare industry is continuously pursuing new and improved medical and technological advancements, treatments, and expanded healthcare opportunities that offer a higher level of patient care. The industry serves a uniquely broad audience, and today’s consumers are searching online for medical providers.
In fact, the majority of consumers rely on the internet to find healthcare providers.
For this reason, Novas says it is critical for medical practices and providers to stay on top of digital marketing strategies and trends in order to keep their organizations competitive and to generate new leads in today’s patient-centric market.
Novas will join healthcare professionals from around the world who specialize in treatments for conditions and diseases affecting aging and elderly patients from around the world for the anti-aging conference in Mexico City later this month.

Instituto de Medicina Antenvejecimiento Longevidad
IX Congress attendees will be granted recognition for their participation and will have access to the event ‘s commercial area where they can purchase products and learn about business opportunities with leading national and international companies specializing in anti-aging medicine.
The conference will be held at Hotel Reforma in Mexico City.
To learn more or register for the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad, visit the conference website. For information on Benito Novas and regenerative and stem cell medicine, visit the Global Stem Cells Group website, email info@stemcellsgroup.com, or call +1 305 560 5337.
About Global Stem Cells Group

Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Benito Novas keynote speaker
- Published in Press Releases
Global Stem Cells Group to Sponsor Congreso Internacional de Medicina Antienvejecimiento y Longevidad Feb. 16-18, 2018
(Image: Hotel Reforma, Mexico City)
MIAMI, Feb. 14, 2018— Global Stem Cells Group, a world leader in stem cell and regenerative medicine, will sponsor the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad (Congress in Anti-Aging and Longevity Medicine) to be held February 16 – 18, 2018 in Mexico City. This will be the third consecutive year GSCG is sponsoring the Congress.
Global Stem Cells Group founder and CEO Benito Novas will join healthcare professionals from around the world for the event. Anti-aging medicine focuses on early detection, prevention, treatment, and regeneration for conditions related to advanced age and promotes. innovative therapy and protocol development for the therapeutic management of aging and elderly patients. Four-hundred participants are expected to attend.

Instituto de Medicina Antenvejecimiento Longevidad
Physicians and medical professionals specializing in age-related diseases and conditions will gather to discuss and exchange knowledge and new treatments in anti-aging medicine. They include: regenerative and stem cell medicine specialists, internists, endocrinologists, dermatologists, geriatricians, surgeons, family and general medicine practitioners, gerontologists, OB/GYN (gynecology and obstetrics), cardiologists, orthopedists, plastic surgeons, chiropractors, dental surgeons, rehabilitative medicine practitioners, sports medicine specialists, homeopathic medicine specialists, nutritionists, pharmaceutical chemists, researchers and , and specialist geriatric nurses.
A lineup of world-renowned physicians, all leaders in anti-aging medicine, will teach magisterial conferences throughout the event. There will also be practical theoretic workshops related to anti-aging, regenerative, and aesthetic medicine. Additionally, conference attendees will be able to witness various procedures performed by expert anti-aging practitioners.
The conference is organized by The Institute of Anti-Aging and Longevity Medicine, the leading anti-aging and regenerative medicine society in Mexico.
IX Congress attendees will be granted recognition for their participation and will have access to the event’s commercial area where they can purchase products and learn about business opportunities with leading national and international companies specializing in anti-aging medicine.
The conference will be held at Hotel Reforma in Mexico City.
To learn more or register for the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad, visit the conference website. For information on regenerative and stem cell medicine, visit the Global Stem Cells Group website, email info@stemcellsgroup.com, or call +1 305 560 5337.
About Global Stem Cells Group
 Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Congress in Anti-Aging and Longevity Medicine
- Published in Press Releases
What is regenerative medicine?
Regenerative medicine is the cutting-edge “medicine of the future,” offering the promise of efficacy by enabling human tissue to be repaired, replaced, and healed (regenerated) once it is damaged or diseased. These therapies supplement the body’s natural healing mechanisms, often employing stem cells to stimulate the renewal of tissue damaged by injury, disease, or age. The rapid expansion of scientific knowledge offers great promise for advances in this field, holding vast potential to improve the quality of human life.
What are Stem Cells?
Stem cells are the basic building blocks of life. They are unspecialized cells capable of producing more stem cells through mitosis or differentiating into specialized cells that perform specific functions in the body. Stem cells are found throughout the body’s tissues, organs, and systems, although usually in small quantities in adults.
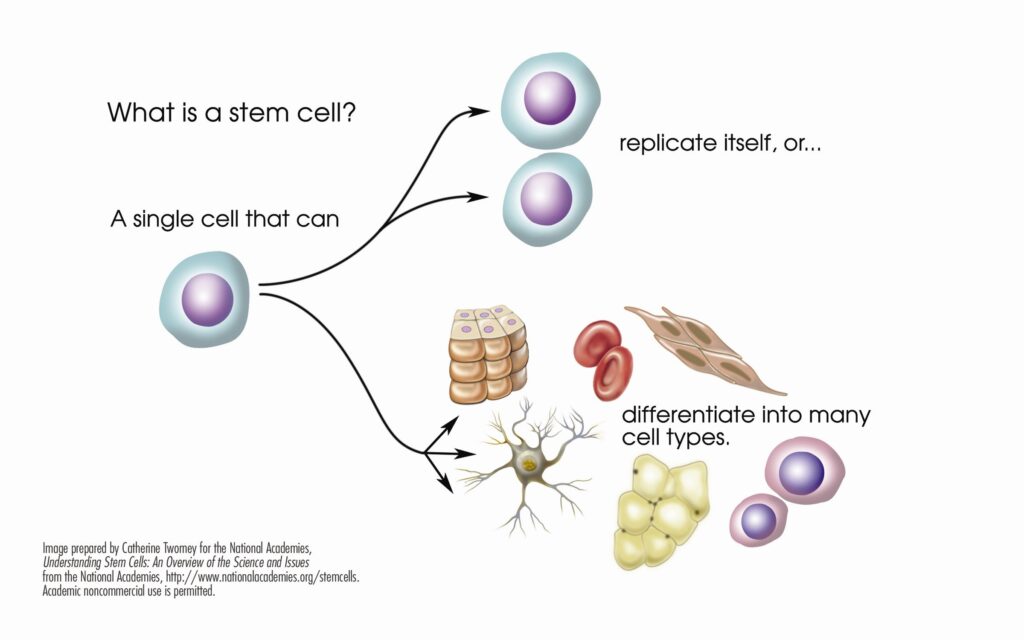
What are Hematopoietic Stem Cells?
Hematopoietic stem cells (HSCs) can give rise to all types of blood cells, including red blood cells, white blood cells, and platelets. They are particularly useful in treating blood-related diseases and conditions.
What are Mesenchymal Stem Cells?
Mesenchymal Stem Cells (MSCs) are multipotent stromal cells that are non-blood forming stem cells capable of differentiating into a variety of cell types, including muscle, bone, cartilage, and fat cells. When introduced into a patient’s body, MSCs can repair or replace damaged or degenerating tissue by communicating with surrounding cells, triggering a cellular cascade of healing (paracrine signaling).
The term MSC was coined in the late 1980s by Dr. Arnold Caplan of Case Western Reserve. Recently, Caplan redefined the acronym to “Medicinal Signaling Cell” to emphasize these cells’ role in secreting powerful bioactive molecules involved in cellular signaling and regeneration. Caplan now describes MSCs as a “multisite-regulatory dispensary” (Natural Drug Store).
The production of MSCs in the human body can be precipitated by bioactive placental tissues containing Growth Factors, Cytokines, and other powerful bioactive agents that trigger cell signaling. The remarkable ability of MSCs makes them irreplaceable in medical treatments.
What are Accessible Sources of Stem Cells?
Stem cells can be extracted from various parts of the body, such as bone marrow and adipose tissue. More recently, birth tissues from live births, including umbilical cord blood, cord tissue with Wharton’s Jelly, and amniotic membrane tissue, have been found to be rich sources of both HSCs and MSCs. These tissues also precipitate the production of MSCs through paracrine signaling. Growth Factors, Cytokines, Exosomes, and micro-RNA from birth tissues give rise to stem cells in this way. These cells, as well as MSCs contained in Wharton’s Jelly, tend to be more fit than those obtained from adult stem cells.
Wharton’s Jelly
Wharton’s Jelly is a gelatinous substance found in the umbilical cord that is rich in stem cells. Studies have shown that mesenchymal stem cells (MSCs) derived from Wharton’s Jelly have low immunogenicity. Human umbilical cord Wharton’s Jelly provides a new source for MSCs that are highly proliferative and have multi-differentiation potential. Wharton’s Jelly Cells (WJCs) express MSC markers but have low levels of human leukocyte antigen (HLA)-ABC and no HLA-DR. WJCs have low functional immunogenicity, and recipient rejection has not been documented.
Advanced Regenerative Medicine
Advanced Regenerative Medicine involves the use of regenerative biomolecules, tissue engineering, and stem cells to treat diseased or injured tissues. This field leverages cutting-edge techniques to promote healing and regeneration, offering new hope for patients with previously untreatable conditions.
- Published in Corporate News / Blog
Controlling Stem Cells… through their environment
Stem cells have captured the imagination of both scientists and the public alike with their potential to revolutionize medical treatments and therapies. The ability of these microscopic entities to differentiate into various cell types holds promise for curing diseases and repairing damaged tissues.
Understanding Stem Cell Differentiation
Researchers are actively exploring methods to manipulate stem cell development, particularly pluripotent cells capable of becoming any cell type. By culturing these cells in specific environments, scientists have observed activation of genes that dictate tissue specialization. This insight is crucial for developing targeted therapies and treatments.
Innovations in Stem Cell Research
Recent breakthroughs include the generation of dopamine-producing neurons from pluripotent cells and the creation of sugar-based scaffolds for blood vessels. These advancements highlight the potential of stem cells to address complex medical challenges.
Clinical Applications of Adult Stem Cells
Physicians have increasingly utilized adult stem cells sourced from fat or bone marrow to treat degenerative conditions such as knee osteoarthritis, sports injuries, and frozen shoulder. Unlike pluripotent cells, adult stem cells primarily function by releasing growth factors, proteins, and anti-inflammatory substances that promote tissue regeneration and healing.
Natural Healing Through Cellular Environment
In clinical settings, adult stem cells influence their environment by enhancing local cell growth and differentiation. This natural process mimics laboratory findings, demonstrating the body’s innate ability to harness its own healing potential.
Conclusion
The field of stem cell research continues to evolve, promising new avenues for medical intervention and patient care. By understanding how stem cells interact with their environment, researchers and healthcare providers can optimize therapies and explore novel treatments for a range of conditions

- Published in Corporate News / Blog
Photobiomodulation and Why We Use LED to Irradiate PRP
Introduction to PRP in Regenerative Medicine
In the regenerative medicine specialty, we have been active for about 8 years. Like many others, we started by using Platelet-Rich Plasma (PRP). We learned about it, were fascinated by it, and treated our patients with it. Patients loved it, and so did we.
PRP, if obtained and used correctly, is a powerful tool to implement in any medical practice, especially when dealing with the elderly, osteoarthritis, wear and tear of tendons and ligaments, and loss of vitality. PRP is also frequently used in plastic and reconstructive surgery for wound care, scar improvement, and overall skin rejuvenation.
How PRP Works
But why does PRP work, and how?
We are all familiar with platelets. They have significant power and influence over tissue regeneration, and by concentrating them in a blood sample, we can obtain signaling proteins, cytokines, and growth factors. If we add white blood cells to the mix, we get what is called L-PRP (leukocyte-rich PRP), making that “soup” even more interesting.
What is Photobiomodulation?
To harness the power of these bioactive substances, we need to coax the cells into releasing them. Normally, platelets are activated by the addition of calcium or by contact with collagen. However, several studies have demonstrated the influence of low-intensity laser on the activity of some cells. This effect is called “photobiomodulation.” This modality uses non-ionizing light sources, like LEDs or Helium-Neon lasers, to produce photochemical events at various biological scales. It has been demonstrated that this light reacts with the enzyme cytochrome c oxidase, which is paramount in mitochondrial processes.
Effects of Photobiomodulation on Cellular Cultures
Several scientists studied this light and its effects on cellular cultures. They found that cells proliferate more when exposed to low-level laser and showed increased viability.
We compared the levels of cytokines and growth factors in an irradiated sample and a non-irradiated one. Some growth factors even tripled in concentration after laser exposure. The famous interleukin-10, an anti-inflammatory protein, doubled in levels, and endorphins were released in high levels.
Benefits of Photobiomodulation
The photobiomodulation process has been established to provide extraordinary benefits in pain management, inflammation reduction, immunomodulation, and promotion of wound healing and tissue regeneration. It plays a fundamental part in our protocols.
Isn’t it all sort of amazing?
We’ll see you in the next blog. Keep your cells healthy!
- Published in Corporate News / Blog
Global Stem Cells Group names Mehmet Veli Karaaltın, M.D. to Advisory Board
(Image: Mehmet Veli Karaaltın, M.D.)
MIAMI, Jan. 18, 2018—Global Stem Cells Group CEO Benito Novas has named Mehmet Veli Karaaltın, M.D., Associate Professor of Plastic, Aesthetic, and Reconstructive Surgery, (European Board Certified), and hand surgery, head, and maxillofacial surgery specialist to the GSCG Advisory Board Faculty.
Karaaltın practices in Istanbul, Turkey.
Born in Iraq-Kirkuk in 1972, Karaaltın moved to the United States with his family, living in West Lafayette, Indiana and Los Angeles until 1988, when he moved to Istanbul.
After graduating from Istanbul University’s Cerrahpaşa English Medical Faculty, Karaaltın received a national examination from Hacettepe University in Ankara, Turkey, where he was 12th out of 25000 doctoral candidates. In 2012, he passed and completed all ID exams required by the European Society of Plastic Surgery. He is a member of the European Council of Plastic Reconstructive and Aesthetic Surgery and the International Society of Aesthetic Plastic Surgery (ISAPS).
In addition to his work in aesthetic applications, Karaaltın is also known for his work in microvascular free flaps, nerve transfers, facial surgery, and tissue transplants.
Karaaltın also practices cellular therapy and tissue regeneration for healing diabetic wounds, severe burns, and Buerger’s disease. He is one of only a few physicians in the world who offer surgical treatment for Lymphedema-Elephant Disease with microvascular lymph node transfer. In addition, Karaaltın uses
3-D technology to perform aesthetic rhinoplasty.
“It is a pleasure to welcome Dr. Karaaltın to the Global Stem Cells Group Advisory Board,” says GSCG CEO Benito Novas. “He brings a wealth of knowledge in aesthetic regenerative medicine to the table, and we look forward to a long and productive alliance.”
To learn more, visit the Global Stem Cells Group website, email inf@stemcellsgroup.com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Mehmet Veli Karaaltın, M.D.
- Published in Press Releases