Global Stem Cells Group’s Benito Novas Announces Agreement with Clinica Planas
The ongoing partnership will offer certification in regenerative medicine for European-based medical practitioners .
MIAMI LAKES, Florida—The International Society for Stem Cell Application (ISSCA), a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine, has signed an agreement with European-based Clinica Planas to offer a stem cells certification training program. The first event will be held in Barcelona on October 18-19, 2019.
“The Global Stem Cells Group is pleased to partner with Clinica Planas to offer this important certification in cell therapies,” said Benito Novas, Vice President of the Global Stem Cells Group. “This partnership will continue our mission to help provide knowledge of regenerative medicine practices and treatment options to physicians across the globe.”
Clinica Planas has two locations in Spain—Barcelona and Madrid—and is known as one of the most prestigious medical centers in Europe. In this new deal, Clinical Planas will partner with the Global Stem Cells Group to help train European-based physicians in the regenerative medicine industry’s best practices and most up-to-date knowledge.
The inaugural certification workshop will be held on October 11-12 in Barcelona, with additional certification opportunities to be offered on an ongoing basis in the future. During the two-day event, physicians will receive insight on 22 proprietary treatment protocols to treat degenerative diseases, acquire skills to be able to offer alternative treatment options to patients who have yet to try regenerative medicine as an option, and join an exclusive network of doctors at the forefront of medical science.
“Our goal is to help physicians looking for new treatment options for their patients suffering from degenerative diseases gain the necessary skills to be able to offer regenerative treatments as an option to alleviate their patients’ suffering,” Novas noted.
To learn more about the October 2019 certification event in Barcelona, visit http://cursocelulasmadre.com/entrenamiento-en-barcelona/.
To learn more about Clinica Planas, visit https://www.clinicaplanas.com/en. To learn more about ISSCA and all of their upcoming and past events, visit https://issca.us/.
- Published in Press Releases
Lans Holdings Enters into Binding LOI to Acquire Global Stem Cells Group
MIAMI LAKES, FL, May 30, 2019-Global Stem Cells Group Inc. (GSCG) is pleased to announce that it has entered into a binding letter of intent to sell 100% to Lans Holdings Inc. (OTCPink: LAHO)
GSCG operates in the global stem cell market which is forecasted by Transparency Market Research to reach $270 billion dollars by 2025 of which roughly 40% or $100 billion will be derived from outside of North America and growing at a 13.8% compounded annual growth rate.
GSCG specializes in leading-edge stem cell research, patient application and physician training. The company licenses its name and intellectual property to physicians around the world and supplies them with necessary equipment and medical supplies to perform stem cell treatments. The company currently has one of the largest member clinic networks in the world spanning over 25 countries.
“We are very pleased to have entered into this binding LOI with Global Stem Cells Group,” said Dave Christensen, CEO of Lans Holdings Inc. “This transaction represents a significant opportunity for our company to enter the explosive field of Stem Cell technology with a proven and seasoned team and I look forward to working closely with GSCG to execute on their growth plans.”
“We are excited to join forces with Lans Holdings,” said Benito Novas, President and CEO of Global Stem Cells Group. “This union will allow us to have access to the capital markets to further fuel our growth as the global stem cells market enters into an accelerated expansion phase.”
- Published in Press Releases
Benito Novas, Global Stem Cells Group CEO, Slated to Give Keynote Speech.
Novas is a global expert on medical practice marketing strategies, specifically those pertaining to regenerative medicine.
MIAMI LAKES, Florida—Benito Novas, CEO of the Global Stem Cells Group, will serve as the keynote speaker at an event sponsored by the International Society for Stem Cell Application held in Mexico City on May 3-4, 2019. Novas is known as a global experts on medical practice marketing strategies, specifically those pertaining to the regenerative medicine field.
The Mexico City Stem Cells Seminar is a two-day medical congress and the first of its kind to offer an entire day focused on medical marketing. Novas will serve as both a keynote speaker and also as a marketing professional to offer his expertise during this portion of the congress.
As the CEO of the Global Stem Cells Group, Benito Novas is an entrepreneur and leader of an international enterprise that provides complete services to the regenerative medicine industry. Though his work, he has created programs that provide Stem Cells Certification opportunities for practitioners in the field in addition to supporting stem cells medical research efforts across the globe.

Benito Novas is known as a global expert on research marketing strategies, especially pertaining to the realm of regenerative medicine. He has extensively researched marketing strategies that are proven to grow medical practices worldwide, including those in the US. Putting his research to use, Novas has successfully grown regenerative medical practices across the globe.
In addition to his personal research pursuits, Benito Novas frequently lends his expertise as a marketing expert to private medical practices and other groups worldwide. He has also conducted medical business in various countries across South America and the Middle East.
To learn more about Benito Novas, visit his LinkedIn profile at https://bit.ly/2IMgGtJ.
- Published in Press Releases
Cancun Stem Cells Center Offers Hope for US Patients Suffering from Degenerative or Chronic Diseases
Free from US regulatory constraints, the center offers stem cells protocols needed to effectively treat a wide variety of disease.
MIAMI LAKES, Florida—For physicians based in the United States looking for high-quality patient care in the realm of regenerative medicine, the Stem Cells Center in Cancun, Mexico offers a cutting-edge care solution outside of the US. The Cancun center is a member of the Global Stem Cells Group network, which is comprised of a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology and regenerative medicine.
A number of conditions that patients present may require physicians to use treatment methods that will increase the cell counts that are delivered during the protocol. For patients suffering from chronic degenerative conditions or disorders based in genetics, physicians may need to culture expanded autologous cells. Currently, autologous stem cell expansion conducted in labs before being reintroduced into patients is not a viable treatment option in the US due to regulatory constraints. Thus, both the patient and physician are left turning to less effective treatment options using only cells obtained in the medical office.
For patients suffering from some degenerative conditions, more involved methods of therapy and more aggressive ways of deploying cells may be necessary than current US regulations allow. To deploy these more aggressive forms of treatment, catheterization is ideal. To perform intricate catheterizations of the carotid artery and the vascular arteries of the brain, for instance, it is critical to have a properly staffed and equipped hospital to perform the procedure. A hemodynamics suite and having an intensive care unit nearby are essential tenets of such treatment protocols.

Patients requiring more aggressive and effective treatment options for degenerative and chronic diseases can receive the treatment they need at the Stem Cell Center in Cancun. Because the facility is located outside of the US, patients and physicians are not bound by regulatory limitations affecting lab-expanded autologous cells.
The Global Stem Cells Groups’ facility in Cancun is a Level 3 hospital equipped with an intensive care operating room and a hemodynamics suite ideal for patients needing critical treatments using regenerative medicine. Hospital staff includes a highly-ranked, high-level specialist in the area of regenerative medicine. Current facility protocols are in place to cater to patients needing the facility’s specialized services, and the hospital is associated with a local laboratory that can easily provide the amount of allogeneic cells needed for treatments requiring them.
Physicians with patients who may benefit from the medical services offered at the Stem Cells Center in Cancun are invited to reach out to set up a meeting to learn more about what services the facility offers and how to access the center for treatment. To learn more about the Global Stem Cells Group and the Cancun facility, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
Which Works Better, Viscosupplementation, or Platelet Rich Plasma?
Introduction to Viscosupplementation
One of the treatments approved by the FDA for knee arthritis is Hyaluronic Acid (HA) injection, commonly known as viscosupplementation. This treatment has shown significant success in managing knee arthritis and is increasingly being explored for other parts of the body, such as the hip and shoulder, although these uses are not FDA-approved. HA injections mimic the fluid that naturally surrounds your joints, acting as a lubricant and shock absorber, thereby reducing arthritis pain. Over time, HA is absorbed into the joint, potentially stimulating the body to produce more stable cartilage.
Evidence Supporting Viscosupplementation
The evidence supporting HA injections is robust. A systematic review of 76 randomized controlled trials concluded that HA injections can improve function, reduce pain, and serve as a reliable treatment for knee osteoarthritis.
The Role of Platelet-Rich Plasma (PRP) in Treating Arthritis
What is Platelet-Rich Plasma (PRP)?
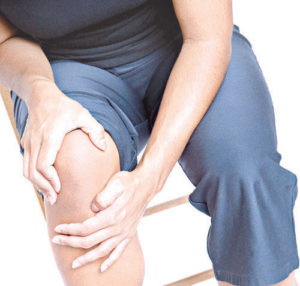
Platelet-Rich Plasma (PRP) is a concentrate of platelets in plasma, containing 6 to 10 times more platelets than normal blood. PRP is rich in growth factors, such as Epidermal Growth Factor (EGF) and Connective Tissue Growth Factor (CTGF), which promote healing by leveraging the body’s natural healing mechanisms.
FDA Regulation and Research on PRP
Unlike HA, PRP is not FDA-regulated, and the devices used to prepare PRP are subject to FDA approval. Despite this, numerous studies have demonstrated PRP’s efficacy in treating tendon injuries and osteoarthritis, as well as in reducing pain. Ongoing research is exploring its potential benefits for hair regrowth, cardiac muscle repair, and dermatologic rejuvenation.
Comparing HA Injections and PRP
Effectiveness and Safety
Studies indicate that PRP can be just as effective, if not more so, than HA injections. While HA is FDA-approved only for knee use, PRP is derived from the patient’s own blood, reducing the risk of infection and autoimmune reactions, thanks to the presence of white blood cells that help fight infections.
Cost Considerations
HA injections for joints other than the knee are not FDA-approved or typically covered by insurance, often costing patients $1500 or more, excluding additional charges like doctor visits and the injection itself. In contrast, PRP treatments generally cost between $800 and $1200 out of pocket.
Conclusion: Which is the Better Choice?
PRP has demonstrated comparable or superior effectiveness to HA injections for arthritis pain management. It carries a lower risk of infection or autoimmune reactions and is generally more cost-effective. Given these advantages, PRP often emerges as the preferred choice for many patients and healthcare providers.
- Published in Corporate News / Blog
Julio Ferreira, M.D.
Julio Ferreira, M.D.
Global Stem Cells Group Advisory Board member
Julio Ferreira, M.D., is an internationally recognized and respected cosmetic surgeon and professor of medicine and aesthetic surgery at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil;
As director of Clinica Ferreira in Argentina, Dr. Ferreira is dedicated to the combination of art and science in aesthetic medicine.
Dr. Ferreira serves as president of the South American Academy of Cosmetic Surgery and expert examiner at the International Board of Cosmetic Surgery. He also serves on the International Editorial Advisory Board of the American Journal of Cosmetic Surgery; former President of the International Academy of Cosmetic Surgery 2005/2007; a member and examiner, International Board of Cosmetic Surgery; Corresponding Fellow of the American Academy of Cosmetic Surgery; Honorary Member of the Spanish Society of Cosmetic Medicine and Surgery; Honorary Member of the Eurorusa Confederation of Societies of Aesthetic Plastic Surgery; Honorary Member of the Bulgarian Society of Cosmetic Surgery; Honorary Member of the Chilean Society of Cosmetic Surgery and Lipoplasty; Honorary Member of the Italian Society of Aesthetic Surgery, and Honorary Member of the French Society of Aesthetic Surgery.
- Published in Advisory Board Members







