GSCG announces an on-site course at OHIO
Global Stem Cells Group (GSCG) has announced a new on-site training course to be conducted through the International Society for Stem Cell Application (ISSCA) in Ohio. This on-site training course will be presented as a total solution to teach and train medical practitioners on the most recent advances in cellular-based treatments and regenerative medicine. It demonstrates the growing demand for clinics and doctors to integrate regenerative medicine into their services.
Personalized Hands-On Training with Industry-Leading Instructors
The new on-site training course to be conducted in Ohio will offer a personalized, hands-on approach, bringing industry leading instructors to the trainees’ clinics. “This course takes a highly visual and interactive angle, which provides trainers with the ability to teach reintegration and procedural techniques on live patients using different protocols for isolating stem cells – right from their own medical establishment,” says Benito Novas, CEO of the GSCG.
The on-site training course will include and offer the following:
Fully Dedicated Instructors
The hands-on approach to this training course involves using industry-leading instructors to offer one-on-one learning. It is the best and most effective way of learning, understanding, and becoming skilled at adipose stem cells and bone marrow extraction procedures.
Staff Training
Doctors usually delegate the process of PRP isolation and obtaining stem cells from bone marrow or fat to their nurses and medical assistants because the procedures are methodical. However, nurses and medical assistants must be fully trained and skilled in these fields. This course imparts them with the relevant skills, enabling them to perform these procedures when doctors need their help.
Trainees’ Preferred Treatment Protocols
This will be a personalized training course, so trainees can choose the treatment protocols they want to learn about and the illnesses they want to target. The instructors will teach not only the process of obtaining cells; they will also reintegrate the cells into the patient and teach about the various reintegration methods based on the patient’s medical conditions.
Equipment & Kits
Isolating and reintegrating stem cells requires the use of certain equipment and kits. The administrators will deliver and install the required equipment, kit, and consumables in the trainees’ clinics.
The Scheduling Process
This course is fully personalized, meaning trainees will have varying needs and preferences. The ISSCA will conduct an initial consultation to discuss the types of treatments trainees want to learn, the patients they need to schedule for treatment, and the underlying costs. The information provided during the initial consultation will enable the ISSCA to gather the relevant supplies, equipment, and training materials for each training session.
About Global Stem Cell Group (GSCG)
Global Stem Cells Group is the parent company of six companies that are dedicated to stem cell research, solutions, and technology training. The group was founded in 2012 and combines dedicated researchers, patient educators, and physician trainers with the shared goal of meeting the need for high-end stem cell solutions and treatments.
Given that the group has a singular focus in this field, it is uniquely positioned to become the global leader in cellular medicine. In addition, by bringing together leading professionals in cellular medicine, it can discover issues that the industry faces and focus its research and development in this area. This specialization has, undoubtedly, enabled it to come up with solutions that address some of the significant issues that most stakeholders are facing in the industry.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
About the International Society for Stem Cell Application (ISSCA)
ISSCA is a multidisciplinary community of physicians and scientists working together to advance science, technology, and the practice of regenerative medicine to treat diseases and lessen human suffering. The group aims to assume a leadership position in regenerative medicine’s research, publication, education, training, and certification standards. ISSCA adopts a hands-on approach by offering certification training worldwide, spreading the practice of regenerative medicine.
- Published in Press Releases
Announcement Of Additional Training Days For Cell therapy Certification in Cancún
The International Society for Stem Cell Application (ISSCA) announced that it would conduct a regenerative medicine certification training course in stem cell therapy in Cancun, Mexico, from 7th to 8th October. Now, the ISSCA is thrilled to announce that the original training course has been sold out. Those still interested in taking this stem cell therapy course will also be glad to know that the ISSCA has scheduled a new opening date: from 9th to 10th October.
This announcement is driven by the growing demand for doctors in Mexico to learn and include stem cell therapy in their services. “We are excited to learn that our upcoming course on cell therapy in Cancun, Mexico has sold out, which shows that more and more physicians want to implement regenerative medicine in their clinics,” says Benito Novas, Founder and CEO of Global Stem Cells Group (GSCG). ISSCA is an educational division of GSCG.
New Opening Date, Same Training Course
There is a new date open to doctors who were not able to register on the original date..The skills and lessons taught remain the same, giving trainees in the second shift access to the same knowledge as those in the original shift.
This two-day course will teach how to harvest bone marrow and adipose stem cells from patients in a clinical setting. It is designed for physicians and high-level practitioners.
The first day will cover the course’s theoretical portion, familiarizing the doctors with the subject. The second day will then cover the course’s practical portion using live cases of various clinical applications of stem cells and exosomes in a clinical setting.
Doctors will learn skills to help them treat their patients more effectively. These skills will also help advance their careers, especially considering the growing importance of stem cell therapy across multiple medical fields. The course will only be available for eight people, so time is of the essence for those who wish to book a spot. Visit https://cursocelulasmadre.com/cursosde-certificacion/cursos-presenciales/ to learn more about the course and register.
About the Global Stem Cells Group (GSCG)
GSCG is a global network of seven major medical corporations exploring various ways of advancing technological and scientific advancements in stem cell development, treatment, and training.
The advancements achieved by GSCG enable it to provide medical practitioners with stem cell solutions that are compliant with the highest medical standards. The company is also publicly traded under the symbol MSSV.
About the International Society for Stem Cell Application (ISSCA)
ISSCA is the educational division of GSCG. It is also a multidisciplinary group of scientists and physicians exploring various ways of advancing the technology, science, and practice of regenerative medicine. The group aims to assume a leadership position in regenerative medicine’s research, publication, education, training, and certification standards. ISSCA has trained more than 10,000 physicians worldwide and adopts a hands-on approach by offering certification training worldwide, spreading the practice of regenerative medicine. Their main goal is to treat diseases more effectively, thereby reducing human suffering.
- Published in Press Releases
Global Stem Cells Group Plans Bone Marrow Clinical Trials for Knee Osteoarthritis
Global Stem Cells Group has announced plans to hold clinical trials, pending IRB approval, for bone marrow stem cell treatments targeting knee osteoarthritis. The trials will be held in five GSCG facilities in the U.S. and South America, with 25 patients accepted for each location.
MIAMI, March 31, 2016—Pending Institutional Review Board (IRB) approval, Global Stem Cells Group, Inc. has announced plans to conduct a multi-center, placebo controlled clinical trial to measure the safety and effectiveness of the intra-articular application of freshly isolated bone marrow stem cells for the treatment of osteoarthritis.
The clinical trials, which will begin July 1, 2016 and run for one year, will be held in Global Stem Cell Group facilities in Buenos Aires, Argentina; Bogota, Colombia; Quito, Ecuador; Miami, Florida and Topeka, Kansas. Each center will accept 25 patients per clinical trial, and patients will receive a bone marrow stem cell injection in one knee and a placebo in the other knee..
 The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
Knee osteoarthritis is a chronic, progressive condition affecting an increasing number of people, especially the elderly and obese. It is characterized by degeneration of the cartilage—the natural cushioning between joints inside the knee.
The condition is the result of the wearing away of cartilage. When this happens, the bones of the joints rub more closely against one another with less of the shock-absorbing benefits of cartilage, resulting in pain, swelling, stiffness and a decreased ability to move.
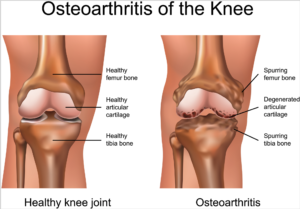 According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
Global Stem Cells Group offers the most advanced protocols and techniques in cellular medicine from around the world.
Details of the protocol and eligibility criteria will be released upon IRB approval.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc, is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces Manufacturing Phase of Progenikine™ SVF Closed System
Global Stem Cells Group has begun the manufacturing phase of Progenikine™, a new SVF closed system kit utilizing EmCyte technology, containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment.
MIAMI, March 31, 2016—Global Stem Cells Group, Inc. has announced that Progenikine™, its new and approved SVF closed system kit using EmCyte technology, is in the manufacturing phase and will be available to physicians in July 2016. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are  isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Developed in conjunction with Patrick Pennie, Emcyte CEO, and and Maritza Novas Director of Research and Development for Global Stem Cells Group, Progenikine  fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
“The Progenikine kit is the newest product designed to help Global Stem Cells Group’s mission to provide accessible products to our member clients, ensuring that more patients will be able to gain access to stem cell therapies,” says Benito Novas, GSCG CEO.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website,email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Emcyte:
Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
###
To view this press release live online, click here
- Published in Press Releases





