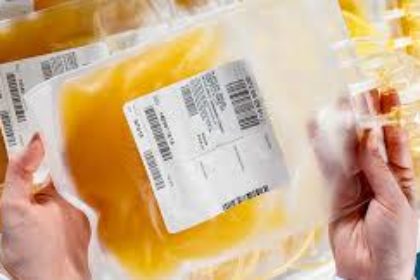
Blood Plasma Reduces Symptoms and Fatalities in Outbreaks of Coronavirus
US blood donation centers are actively collecting plasma from COVID-19 survivors to treat current patients infected with the disease. Convalescent plasma therapy, an old treatment method revived for COVID-19, involves using blood products from recovered patients to inject into those still battling the virus. This approach, historically used as far back as the 1930s for diseases like measles and more recently for H1N1 influenza, SARS, and Ebola, has shown promise in reducing symptoms and fatalities during past coronavirus outbreaks.
History and Effectiveness of Convalescent Plasma Therapy
Clinical research has demonstrated that convalescent plasma can provide hope in treating COVID-19 patients by temporarily boosting their immune response until they develop their own antibodies. This therapy has gained attention due to its potential to mitigate severe symptoms in the absence of specific treatments or vaccines.
Current Efforts and Clinical Trials
Following FDA authorization for emergency use, centers like Houston Methodist Hospital have started administering convalescent plasma to critically ill COVID-19 patients. Ongoing clinical trials across various locations, including the New York Blood Center and American Red Cross, aim to validate the effectiveness of plasma therapy in combating the pandemic.
Guidelines and Collection Process
According to AABB guidelines, plasma is collected from donors who have recovered from COVID-19, tested negative, and been symptom-free for at least 28 days. Efforts are underway to streamline antibody testing for donors and quickly administer plasma to critically ill patients to maximize its therapeutic potential.
Future Prospects and Collaborative Efforts
Collaborations between AABB, America’s Blood Centers, and medical institutions are expected to expand plasma therapy practices exponentially, facilitating broader access and implementation. Emergency IND authorization from the FDA allows for the immediate distribution of collected plasma to patients, marking a significant step in pandemic response efforts.
Personal Stories and Community Engagement
Individuals like Dr. Jon Peters, a family practice physician recovering from COVID-19, are eager to contribute by donating plasma once they meet recovery criteria. Such donations are critical in providing a low-risk, potentially lifesaving treatment option for those affected by the pandemic.
To learn more about convalescent plasma therapy for COVID-19 and participate in ongoing research efforts, visit www.issca.us and stay updated on the latest developments in combating the coronavirus pandemic.