Adimarket announces launch of Cellgenic stem cell protein extract hair restoration product
GSCG affiliate Adimarket announces the launch of the Cellgenic adipose-derived stem cell hair restoration application that maximizes the skin and hair follicles’ own revitalizing capabilities.
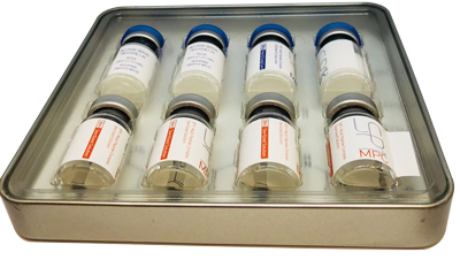
MIAMI, June 26, 2018—Global Stem Cells Group (GSCG) affiliate Adimarket announces the launch of the Cellgenic adipose-derived stem cells protein extract (CADPE) hair restoration application product.
CADPE combines refined growth factor proteins extracted from human adipose-derived stem cells in a conditioned media and a unique protein formula, to maximize the revitalizing characteristic of skin and hair follicles.
CADPE increases the reproduction of human follicles’ dermal papilla cells, which consist of a distinct population of specialized fibroblasts important in morphogenesis (differentiation and growth) of the hair follicle structure and control of the hair growth cycle, CADPE works by turning over dying skin cells at twice the frequency of normal skin,
CADPE benefits:
• Use of active proteins derived from human sources eliminates concerns with allergic reactions and skin irritation
• The natural composition of the product’s various growth factors acquired from adipose-derived stem cells can provide exceptional biological activity
• CADPE is multi-functioning, exhibiting anti-oxidation and hair re-growth effects
• Each growth factor has a pharmacological effect, but the combination of growth factors has a synergistic effect that can enhance the pharmacological action of single proteins.
• No special training is needed to learn how to use CADPE
• The CADPE application procedure is shorter than other hair restoration protocols, which can take up to eight hours per patient
• CADPE is supported by scientific evidence
When used by aesthetic practitioners, CADPE provides safe, cutting-edge hair thickening and hair follicle proliferation results that are CTFA-approved, take less application time than other hair restoration processes, and is backed by medical research.
Cellgenic adipose-derived stem cells protein extract can be purchased online at the Adimarket online store. For more information, visit the Adimarket website, email info@stemcellsgroup.com, or call 305-560-5337.
About Adimarket:

Adimarket was founded to provide practitioners the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting-edge treatments.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines 
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
CADPE stem cell hair restoration application
- Published in Press Releases
Global Stem Cells Group to sponsor the American Association of Stem Cell Physicians summit in Miami
Global Stem Cells Group will sponsor the American Association of Stem Cell Physicians summit in Miami August 10 – 12, 2018.
MIAMI, June 18, 2018—Global Stem Cells Group (GSCG), a world leader in regenerative medicine, announces its sponsorship of the American Association of Stem Cell Physicians (AAOSCP) summit in Miami, August 10-12, 2018. The conference will offer three days of educational, workshop, and networking events with physicians and researchers from around the world in the fields of stem cell and regenerative medicine. Attending physicians can earn up to 24 CME credits.
Summit attendees will have the opportunity to learn about Adimarket, GSCG’S fast-growing online store for regenerative medicine practitioners to conveniently purchase products, supplies, and equipment that meets their specific clinical needs.
GSCG affiliate Stem Cell Training, Inc. (SCT) will also be on hand to provide qualified Summit attendees with personalized, hands-on regenerative medicine training course at the summit, which will be conducted by expert instructors who guide qualified physicians through the intensive, two-day certification program. Benefits of this stem cell training course include:
- Stem Cell Training’s state-of-the-art, one-on-one regenerative medicine training on the latest stem cell procedures and protocols to use immediately in private clinical practice
- Access to SCT’s online resources, personalized theoretical information, and hands-on training with live patients, as well as ongoing support for each participating physician’s clinical practice
- Stem cell applications and protocols demonstrated by an SCT faculty member with extensive experience in laboratory and clinical practices
Physicians and businesses professionals in the field of regenerative medicine, the AAOSCP summit offers an outstanding opportunity to connect with others in the industry, explore, learn, and make connections while learning about the latest groundbreaking treatments and technologies. Medical students and those seeking careers on the business side of the regenerative medicine market can take advantage of the valuable opportunity the summit offers to network with potential employers while learning about groundbreaking treatments and therapies.
Since 2014, Global Stem Cells Group has worked with some of the most prestigious regenerative medicine practitioners in the U.S., South America, and Asia as it focuses on growing its services throughout the global community. Stem cell therapies continue to revolutionize the healthcare industry while offering new hope for sufferers of chronic, debilitating conditions.
For more information and to register for the summit, visit the AAOSCP website, email info@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
American Association of Stem Cell Physicians summit
- Published in Press Releases
ISSCA to sponsor XX International Congress of Aesthetic Medicine, Aesthetic Surgery and Obesity in Mexico City
ISSCA will sponsor the XX International Congress of Aesthetic Medicine, Aesthetic Surgery and Obesity in Mexico City July 13, 14 and 15, 2018. This will mark the international stem cell organization’s third consecutive year sponsoring the event.
MIAMI, June 18, 2018—The International Society for Stem Cell Application (ISSCA), will sponsor the XX International Congress of Aesthetic Medicine, Aesthetic Surgery and Obesity in Mexico City July 13, 14 and 15, 2018. The event marks ISSCA’s third consecutive year of sponsorship for the event, expected to attract aesthetic and regenerative medicine physicians from around the world.
ISSCA affiliate Global Stem Cells Group (GSCG) will launch its newest regenerative medicine products for the aesthetic market and present master classes for Congress attendees in clinical stem cell applications digital marketing for aesthetic practices:
• Clinical Stem Cell Applications master class conducted by GSCG Research Director Maritza Novas, stem cell application and anti-aging specialist.
• Social Media marketing master class Digital Marketing: A New Challenge to the Aesthetic Market, conducted by Benito Novas, Global Stem Cells Group CEO, and medical marketing specialist.
Global Stem Cells Group will also launch its Cellgenic Hair adipose-derived stem cell (ADSC) proteins extract (AAPE). Containing a mixture of refined growth factor proteins extracted from human stem cells, AAPE’s conditioned media offers a unique protein formula that maximizes the revitalizing capabilities in skin and hair follicles.
Key factors of AAPE include:
- Human proteins
- Active proteins are derived from human sources with no risk of allergic reactions or skin irritation
- The natural composition of assorted growth factors derived from ADSCs can provide the best biological activity
- Synergistic effect: each growth factor has a unique pharmacological effect, and the combination AAPE’s of growth factors may enhance the pharmacological action of a single protein.
- Multi-function: AAPE demonstrates anti-oxidation, skin whitening, and hair regrowth capabilities.
The conference will be held at the Hotel Fiesta Americana Reforma in Mexico City.
To learn more about registering to attend, please visit the XX International Congress of Aesthetic Medicine, Aesthetic Surgery, and Obesity website, email mailto:info@stemcellsgroup.com, or call 305-560-5337.
About ISSCA:

The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines
Global Stem Cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
XX International Congress of Aesthetic Medicine, Aesthetic Surgery and Obesity
- Published in Press Releases
What’s all the fuss about Regenerative Medicine?
In popular media, the term Regenerative Medicine, or Stem Cell Therapy, are becoming buzz words. This is because the field of medicine and healthcare is expanding and advancing every day, and many new treatments for otherwise common ailments are being discovered. These conditions range from burns, joint pain, strain, and pretty much every other common ailment out there
Many patients have given up hope with trying to find traditional medicines that work. This is why many people are flocking to try Regenerative Medicine. This is also something that many people who are into holistic healing are trying, as it is simply the body working to heal itself.
Regenerative Medicine works as it takes a sample of your own blood, bone marrow, and other tissues, and then it goes through a process in which to take out a certain material known as Platelet-Rich Plasma. This PRP is then applied to the infected area, so that your body’s own platelets can work to heal your body back to full health, without having to worry about any invasive surgeries.
A good question to ask is why our body does this do this itself. Well, this is because research has shown that by isolating them, they activate, and as a result when injected back into the body start to work harder to fix the issues, such as in a joint, or helping to relieve pain. Many patients who try it say they have gotten good results from the treatment.
Many doctors predict that this therapy will help physicians provide a more non-intrusive treatment that has fewer side effects, and can be big within the coming years. Many compare it to the invention of penicillin with how important it is. It is even growing in popularity with many physicians using training courses to help their patients, leaving many of them happier and healthier.
- Published in Corporate News / Blog
ISSCA to conduct second stem cell certification training course in Lima, Peru
ISSCA to conduct a second hands-on regenerative medicine certification training course in Lima, Peru, Peru August 31, 2018.
MIAMI, June 7, 2018—In response to the success of a recent stem cell certification course in Lima, Peru, the International Society for Stem Cell Application (ISSCA) has announced plans to hold a second stem cell certification training course for qualified physicians in Lima on August 31, 2018.
ISSCA, the only stem cells organization able to travel around the world to teach stem cell harvesting protocols to physicians, conducted the first course in Lima in May. Eight Peruvian physicians were provided highly personalized, hands-on training in harvesting and isolating adipose- and bone marrow-derived stem cells from four live patients under the guidance of stem cell training experts.
Each participating physician obtains the intellectual property of 22 proprietary protocols that will them to treat degenerative and aesthetic diseases and conditions in their offices. Step-by-step videos of each protocol are provided to physicians for later referral.
Participating physicians acquire the skills necessary to offer an alternative therapy to patients with medical conditions for which no solution is currently available. ISSCA’s stem cell training course allows qualified physicians who earn certification to offer sought-after alternative treatments.
Successful completion of the ISSCA regenerative medicine certification course allows physicians to join a select group of practitioners at the forefront of medical science. Only 5 percent of physicians worldwide access to stem cell therapy studies, and so far only 0.01 percent are practicing these therapies.
The course also provides participating physicians with access to ISSCA’s online stem cell training course to review all content and procedures introduced during the two-day clinical training course, as well as patient forms and guidelines, procedures, informed consent forms, didactic lectures, training booklets, and more,
The ISSCA’s regenerative medicine protocols training course was developed for physicians and high-level practitioners to learn techniques in harvesting and reintegrating stem cells derived from patients’ adipose tissue and bone marrow.
Seating is limited to eight participants. Register today at the Lima Peru course website to secure a seat, email info@stemcellsgroup.com, or call 305-560-5337.
About ISSCA:
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made in the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
Stem cell training Lima Peru
- Published in Press Releases
ISSCA to conduct hands-on regenerative medicine training course at its Abu Dhabi Stem Cell Center
ISSCA will conduct a hands-on regenerative medicine certification training course for physicians at its Stem Cell Center in Abu Dhabi July 8, 2018.
MIAMI, June 7, 2018—In an effort to consolidate its presence in the Middle East and focus on providing the best instruction to new regenerative medicine practitioners, the International Society for Stem Cell Application (ISSCA) has announced plans to conduct a hands-on regenerative medicine certification training course for physicians at its Stem Cell Center in Abu Dhabi July 8, 2018.
The intensive, hands-on training course teaches participating physicians how to harvest stem cells from adipose and bone marrow tissue. Qualified physicians will learn as they conduct regenerative medicine protocols on live patients under the direction of stem cell training experts. Skills learned in the training course can be used in the physician’s practice to treat patients and for career advancement opportunities.
The course also provides participating physicians with access to ISSCA’s online stem cell training course to review all content and procedures introduced during the two-day clinical training course, as well as patient forms and guidelines, procedures, informed consent forms, didactic lectures, training booklets, and more.
The ISSCA’s regenerative medicine protocols training course was developed for physicians and high-level practitioners to learn techniques in harvesting and reintegrating stem cells derived from patients’ adipose tissue and bone marrow.
The July 8, 2018, regenerative medicine training course will be held at the ISSCA Stem Cell Center in Abu Dhabi.
Seating for this training course is limited. Register today to participate by visiting the Stem Cell Training Abu Dhabi website, email info@stemcellsgroup.com, or call 305-560-5337.
About ISSCA:
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
Stem cell training Abu Dhabi
- Published in Press Releases
Global Stem Cells Group Announces the First International Meeting on Marketing in Aesthetic Medicine
Global Stem Cells Group announces the first international meeting on marketing in aesthetic medicine August 24, 2018 in Buenos Aires, Argentina.
MIAMI, April 19, 2018—Global Stem Cells Group founder Benito Novas announces the first international meeting on marketing in aesthetic medicine, to be held at the Medicine Faculty of Universidad de Buenos Aires August 24, 2018.
Novas, a global entrepreneur and medical marketing strategist in the fields of biotechnology, life sciences, and healthcare development, will lead a team of experts who will share strategies for marketing an aesthetic medicine practice.
The meeting agenda will provide attendees with tools and insights for promoting their practices, with topics including:
- How to manage social media to recruit more patients
- How to use Instagram and email marketing
- How to use content marketing to attract target audiences
- Promoting an aesthetic clinic in the digital era
- Influencer marketing
Novas, CEO of Global Stem Cells Group and Aesthetic Marketing Group, will be the event’s keynote speaker. He will share his expertise in digital marketing, the latest marketing tools for managing an aesthetic medicine practice, and other strategies for promoting physician practices.
“Consumers spend at least three hours per day on social media sites, which makes social media a valuable tool for attracting patients,” Novas says. “Physicians and medical practice administrators who participate in this meeting can learn how to leverage the power of online resources like Twitter, Facebook, and Instagram to reach target audiences, increase leads, and effectively recruit new patients.
Attendees will also learn about consumer behavior patterns in their search for products and services in the digital age. Most potential patients search Google to find an aesthetic medicine practice that offers the products and procedures they are looking for.
Content marketing and other strategies presented at this meeting will help aesthetic practices maximize opportunities to attract potential patients by enhancing online visibility and increasing engagement with target audiences.
Additional speakers will include:
- Hector Portilla, communications and advertising specialist and director of Medestica Digital Portal, who will discuss marketing strategies
for the development of cosmetic and aesthetic medicine centers - Tamara Paez, Espana, business consultant and author of Marketing Digital en su clinica Estetica (co-authored by Novas)
- Andrea Lapeire, plastic surgeon, Argentina
- Dario Parada, owner, and founder of Grupo NOTO S.A., Argentina
- Alex Novas, Chief Marketing Officer, Global Stem Cells Group U.S.
To learn more about attending Global Stem Cells Group’s first international meeting on marketing in aesthetic medicine email info@stemcellsgroup.com, or send a text via WhatsApp to +1 786 238 2170
About Global Stem Cells Group:

Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Marketing for aesthetic medicine practices
- Published in Press Releases
ISSCA to Host Stem Cell Training Certification Course August 25-26, 2018 in Buenos Aires, Argentina
ISSCA will conduct the next hands-on regenerative medicine certification training course for physicians in Buenos Aires, Argentina, August 25-26. 2018.
MIAMI, April 19, 2018—Following the success of its stem cell training course in Buenos Aires April 6-7, the International Society for Stem Cell Application (ISSCA) has announced plans to host the next hands-on regenerative medicine certification training course for physicians in Buenos Aires August 25-26, 2018.
Eight physicians participated in the April training course, earning certification for harvesting and implanting adipose and bone marrow stem cells in a clinical setting to treat a variety of diseases and conditions including arthritis and osteoarthritis.
Participants learn while they conduct regenerative medicine protocols on live patients under the direction of stem cell training experts. Skills learned in the training course can be used in the physician’s practice for medical and aesthetic treatments and help physicians looking for career advancement opportunities.
Argentinian sports medicine specialist, Damian Ariel Siano M.D., spoke about the benefits of stem cell certification during the training session.
“Now I can offer all my patients a non-surgical option that allows them to avoid surgery for procedures like knee and hip replacement,” Siano says. “For professional athletes, this procedure provides faster recovery and less downtime following the procedure.”
The course also provides participating physicians with access to ISSCA’s online stem cell training course to review all content and procedures introduced during the two-day clinical training course, as well as patient forms and guidelines, procedures, informed consent forms, didactic lectures, training booklets, and more.
The ISSCA’s regenerative medicine protocols training course was developed for physicians and high-level practitioners to learn techniques in harvesting and reintegrating stem cells derived from patients’ adipose tissue and bone marrow.
Stem cell therapies continue to revolutionize the medical industry and help improve the quality of life for patients suffering from sports injuries, age-related conditions, and other chronic ailments.
The August 25-26. 2018 training course will be held at the Medicine Faculty of Universidad de Buenos Aires.
Seating for this training course is limited. Register today to participate by visiting the Stem Cell Training Buenos Aires website, email info@stemcellsgroup.com, or call 305-560-5337.
About ISSCA:

The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
Stem cell training certification Buenos Aires
- Published in Press Releases
Aesthetic Practice Digital Marketing, Regenerative Medicine Master Classes at Jornadas Mediteraneas
ISSCA to host regenerative medicine symposium during Jornadas Mediteraneas—Mediterranean Days of Therapeutic Confrontations in Medicine and Cosmetic Surgery—in Barcelona.
MIAMI, April 2, 2018—The International Association for Stem Cell Application (ISSCA) has announced plans to host a regenerative medicine symposium during Jornadas Mediteraneas—Mediterranean Days of Therapeutic Confrontations in Medicine and Cosmetic Surgery— in Barcelona, Spain May 13, 2018.
The ISSCA symposium will provide a platform for Global Stem Cells Group to conduct master classes in digital marketing for aesthetic practices and regenerative medicine, conducted by specialists in these fields.

Benito Novas, Global Stem Cells Group CEO
Novas’s class will provide aesthetic medicine professionals with a valuable opportunity to learn about the most effective marketing resources and methods for growing an aesthetic medicine practice and best practices for managing different advertising resources.
In addition, Novas’s class will offer expert tips for using content marketing strategies to build a practice. Following the class, Novas will launch his newest book, “Marketing Digital en su clinica Estetica”(“Digital Marketing in Your Aesthetics Clinic”) as well as his new online course, “Digital Marketing for Aesthetic Physicians.

DRA Maritza Novas
Novas and Mesen will offer instruction on adult stem cells, which have attracted the attention of scientists and physicians worldwide for their unique biological properties and potential for treating disease, injuries, and medical conditions. Stem cell research is complex and fast-growing, with new developments progressing rapidly.
Novas will share her expertise, experiences, and perspective on a broad range of topics regarding advancements in mesenchymal stem cell (MSC) applications during their transition from bench side to bedside.

Leslie Mesen, M.D.

DRA Mar Vargas, Lasar Sculpture Medical Clinic
To learn more about the symposium and digital marketing masterclass and to make a reservation, visit the stemcellconference.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About ISSCA:
 The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training. and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical
 corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Jornadas Mediteraneas master classes
- Published in Press Releases
ISSCA President Deyoung Kim, Ph.D., Announces Regenerative Medicine Symposium in Korea Nov. 24, 2018
ISSCA Founder and President Daeyong Kim, Ph.D., announces plans to host the Regenerative Medicine Cell Therapies Symposium in Seoul, Korea in November.
MIAMI, April 2, 2018—International Association for Stem Cell Application (ISSCA) President Daeyong Kim, Ph.D., has announced plans to host Applications of Cell Therapies in Medicine and Aesthetic Surgery, a regenerative medicine symposium, in Seoul Korea November 24, 2018.
The symposium will be ISSCA’s principal event for 2018, during which the organization will launch its entire 2019 program, including stem cell certification courses for physicians to be held in locations worldwide, as well as the 2019 regenerative medicine symposium agenda.
In 2019, ISSCA plans to expand its activities to Asia by hosting at least one regenerative medicine symposium and four stem cell certification courses during the year. The Korea symposium will be structured as a scientific meeting in which regenerative medicine practitioners share their clinical experience. ISSCA affiliate Global Stem Cells Group, and biomedical and leading lab equipment manufacturer N-Biotek will launch new technology for regenerative medicine practitioners.
The international symposium is part of ISSCA’s mission to support a paradigm shift in healthcare from traditional to regenerative medicine in the 21st Century and provide cutting-edge information on developments in all areas of stem cell research. The Buenos Aires event will host a group of renowned international speakers in the field of stem cell and regenerative medicine, who will offer a day of rigorous scientific discourse aimed at physicians.
Topics of focus at the symposium include:
- Management of aging at the cellular level
- Stem cell therapies in medical aesthetics: the latest methods of harvest and isolation Non-invasive protocols of non-surgical facial and body rejuvenation
- Beyond fillers and toxins
- Combined treatment plans that include surgical methods for the management of advanced aging
- Restoration and assisted hair transplantation with biomaterials and growth factors
- The invaluable role of biological cosmeceutical in the management of aging
- The aging process from the inside out: the role of hormones
- Management of the latest digital marketing tools for recruiting patients in their aesthetic clinics
The symposium will incorporate the biology, medicine, applications, regulations, product development, and commercialization of stem cells. Business opportunities, challenges, and potential strategies for overcoming these challenges will also be addressed.
To learn more about the ISSCA Korea symposium and to make a reservation, visit the stemcellconference.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About ISSCA:

The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training. and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
ISSCA Korea regenerative medicine symposium
- Published in Press Releases